Molecular mechanism of enantioselective proton transfer to carbon in catalytic antibody 14D9
- PMID: 14988504
- PMCID: PMC376184
- DOI: 10.1073/pnas.0400263101
Molecular mechanism of enantioselective proton transfer to carbon in catalytic antibody 14D9
Abstract
Catalytic antibody 14D9 catalyzes the enantioselective protonation of prochiral enol ethers with high enantioselectivity (>99% ee) and a practical turnover (k(cat) = 0.4 s(-1)), allowing for preparative scale applications. This antibody represents one of the rare examples of catalytic antibodies promoting acid-catalyzed processes. Antibody 14D9 was cloned and expressed as a chimeric Fab fragment in Escherichia coli. Crystal structures of Fab 14D9 as apo form and of its close analog 19C9 in complex with the transition state analog were determined at 2.8-A resolution. A series of site-directed mutagenesis experiments was carried out to probe the role of individual active-site amino acids. Proton transfer to carbon is catalyzed by a hydrogen bond network formed by the side chains of Asp(H101) and Tyr(L36) with a water molecule serving as a relay. The intermediate oxocarbonium ion formed during the protonation step is trapped by the same water molecule, resulting in an overall syn-addition of water to the enol ether's double bond. The enantioselectivity is caused by steric crowding at the active site, mainly because of the side chain of Phe(H84). The 20-fold lower activity of 19C9 compared with 14D9 was traced down to residue Thr(L46), which forms a nonproductive hydrogen bond with the catalytic residue Asp(H101), which competes with the critical Asp(H101)-Tyr(L36) hydrogen bond and therefore reduces catalytic efficiency. The catalytic activity of 19C9 was restored to that of 14D9 by using either site-directed mutagenesis (Thr(L46)Ala) or chain shuffling.
Figures





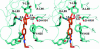
Similar articles
-
Mechanistic study of proton transfer and hysteresis in catalytic antibody 16E7 by site-directed mutagenesis and homology modeling.Bioorg Med Chem. 2005 Feb 15;13(4):1021-9. doi: 10.1016/j.bmc.2004.11.041. Bioorg Med Chem. 2005. PMID: 15670909
-
Antibody catalysis of pericyclic reactions.Acta Chem Scand (Cph). 1996 Apr;50(4):328-32. doi: 10.3891/acta.chem.scand.50-0328. Acta Chem Scand (Cph). 1996. PMID: 8639375
-
Structural origins of efficient proton abstraction from carbon by a catalytic antibody.Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):4984-9. doi: 10.1073/pnas.0409207102. Epub 2005 Mar 23. Proc Natl Acad Sci U S A. 2005. PMID: 15788533 Free PMC article.
-
Pyridoxal-5'-phosphate-dependent catalytic antibodies.J Immunol Methods. 2002 Nov 1;269(1-2):99-110. doi: 10.1016/s0022-1759(02)00227-2. J Immunol Methods. 2002. PMID: 12379355 Review.
-
Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: a perspective from FTIR difference spectroscopy.Biochim Biophys Acta. 2008 Oct;1777(10):1229-48. doi: 10.1016/j.bbabio.2008.06.012. Epub 2008 Jul 11. Biochim Biophys Acta. 2008. PMID: 18671937 Review.
Cited by
-
An efficient one-step site-directed and site-saturation mutagenesis protocol.Nucleic Acids Res. 2004 Aug 10;32(14):e115. doi: 10.1093/nar/gnh110. Nucleic Acids Res. 2004. PMID: 15304544 Free PMC article.
-
RAP Tag and PMab-2 Antibody: A Tagging System for Detecting and Purifying Proteins in Plant Cells.Front Plant Sci. 2020 Sep 10;11:510444. doi: 10.3389/fpls.2020.510444. eCollection 2020. Front Plant Sci. 2020. PMID: 33013955 Free PMC article.
-
Microbial Enzyme Biotechnology to Reach Plastic Waste Circularity: Current Status, Problems and Perspectives.Int J Mol Sci. 2023 Feb 15;24(4):3877. doi: 10.3390/ijms24043877. Int J Mol Sci. 2023. PMID: 36835289 Free PMC article. Review.
-
Conformational adaptability determining antibody recognition to distomer: structure analysis of enantioselective antibody against chiral drug gatifloxacin.RSC Adv. 2021 Dec 13;11(62):39534-39544. doi: 10.1039/d1ra07143b. eCollection 2021 Dec 6. RSC Adv. 2021. PMID: 35492441 Free PMC article.
References
-
- Kemp, D. S. (1995) Nature 373, 196-197. - PubMed
-
- Mitra, B., Kallarakal, A. T., Kozarich, J. W., Gerlt, J. A., Clifton, J. G., Petsko, G. A. & Kenyon, G. L. (1995) Biochemistry 34, 2777-2787. - PubMed
-
- Davenport, R. C., Bash, P. A., Seaton, B. A., Karplus, M., Petsko, G. A. & Ringe, D. (1991) Biochemistry 30, 5821-5826. - PubMed
-
- Stevenson, J. D. & Thomas, N. R. (2000) Nat. Prod. Rep. 17, 535-537. - PubMed
-
- Shokat, K. M., Leumann, C. J., Sugasawara, R. & Schultz, P. G. (1989) Nature 338, 269-271. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

