Cell proliferation and apoptosis in rat mammary cancer after electrochemical treatment (EChT)
- PMID: 14990326
- PMCID: PMC7129577
- DOI: 10.1016/j.bioelechem.2003.10.008
Cell proliferation and apoptosis in rat mammary cancer after electrochemical treatment (EChT)
Abstract
Background: Several authors have recently reported encouraging results from Electrochemical treatment (EChT) in malignant tumours. However, EChT is not established and mechanisms are not completely understood. In vivo studies were conducted to evaluate the toxic changes and effectiveness of EChT on an animal tumour model.
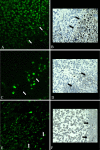
Methods: Tumours were induced by injecting cells from the R3230AC rat mammary tumour cell line clone D subcutaneously, in 28 female Fischer 344 rats. EChT was conducted by inserting a platinum electrode into the tumours. The positive and negative control groups were subjected to the same conditions but without current. The rats were kept for 0, 7 or 14 days post-treatment. Three hours prior to euthanasia an i.p. injection of Bromodioxyuridine (BrdU) was given. The rats were euthanized, the lesions extirpated and samples were collected for histopathological, and immunohistochemical examination.
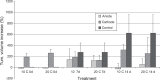
Results: Significant changes in cell proliferation rate were seen both in the cathode and anode regions. Apoptosis were induced in the anodic treated area outside the primary necrosis, detected with the TUNEL method.
Discussion: The results suggest that secondary cell destruction was caused by necrosis with cathodic EChT and apoptosis or necrosis with anodic EChT.
Figures




References
-
- T. Cavallo, A complete treatise on electricity in theory and practice, Dilly, London, 1777.
-
- Humphrey C.E., Seal E.H. Biophysical approach toward tumor regression in mice. Science. 1959;130:388–390. - PubMed
-
- Schauble M.K., Habal M.B., Gullick H.D. Inhibition of experimental tumor growth in hamsters by small direct currents. Arch. Pathol. Lab. Med. 1977;101:294–297. - PubMed
-
- Habal M.B. Effect of applied dc currents on experimental tumor growth in rats. J. Biomed. Mater. Res. 1980;14:789–801. - PubMed
-
- Morris D.M., Marino A.A., Gonzalez E. Electrochemical modification of tumor growth in mice. J. Surg. Res. 1992;53:306–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

