Temperature-dependent conformational transitions and hydrogen-bond dynamics of the elastin-like octapeptide GVG(VPGVG): a molecular-dynamics study
- PMID: 14990469
- PMCID: PMC1303977
- DOI: 10.1016/S0006-3495(04)74210-1
Temperature-dependent conformational transitions and hydrogen-bond dynamics of the elastin-like octapeptide GVG(VPGVG): a molecular-dynamics study
Abstract
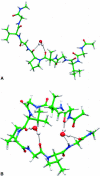
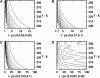
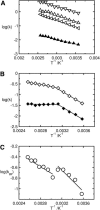
A joint experimental/theoretical investigation of the elastin-like octapeptide GVG(VPGVG) was carried out. In this article a comprehensive molecular-dynamics study of the temperature-dependent folding and unfolding of the octapeptide is presented. The current study, as well as its experimental counterpart (see companion article in this issue) find that this peptide undergoes an inverse temperature transition (ITT), leading to a folding at approximately 40-60 degrees C. In addition, an unfolding transition is identified at unusually high temperatures approaching the normal boiling point of water. Due to the small size of the system, two broad temperature regimes are found: the ITT regime at approximately 10-60 degrees C and the unfolding regime at approximately T > 60 degrees C, where the peptide has a maximum probability of being folded at T approximately 60 degrees C. A detailed molecular picture involving a thermodynamic order parameter, or reaction coordinate, for this process is presented along with a time-correlation function analysis of the hydrogen-bond dynamics within the peptide as well as between the peptide and solvating water molecules. Correlation with experimental evidence and ramifications on the properties of elastin are discussed.
Figures






References
-
- Aalten, D. V., B. D. Groot, J. Findlay, H. Berendsen, and A. Amadei. 1997. A comparison of techniques for calculating protein essential dynamics. J. Comp. Chem. 18:169–181.
-
- Aaron, B., and J. Gosline. 1980. Optical properties of single elastin fibres indicate random protein conformation. Nature. 287:865–867. - PubMed
-
- Amadei, A., A. Linssen, and H. Berendsen. 1993. Essential dynamics of proteins. Proteins Struct. Funct. Gen. 17:412–425. - PubMed
-
- Andriciolaei, I., and M. Karplus. 2001. On the calculation of entropy from covariance matrices of the atomic fluctuations. J. Chem. Phys. 115:6289–6292.
-
- Arad, M. G. O. 1990. Depsipeptide analogues of elastin repeating sequences: conformational analysis. Biopolymers. 29:1651–1668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

