The effect of liposome size on the final lipid/DNA ratio of cationic lipoplexes
- PMID: 14990482
- PMCID: PMC1303990
- DOI: 10.1016/S0006-3495(04)74223-X
The effect of liposome size on the final lipid/DNA ratio of cationic lipoplexes
Abstract
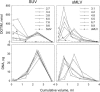
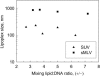
Several studies have demonstrated that lipoplexes are two-phase systems over most mixing lipid/DNA charge ratios. Because these studies have focused on small unilamellar vesicles (SUV), they leave open the question as to whether a similar pattern is followed by other liposome types. The main purpose of this work is to examine the question further by characterizing the assembly of cationic lipoplexes prepared from 1-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl)imidazolinium chloride (DOTIM)/dioleoylphosphatidylethanolamine (DOPE) (1:1) liposomes of various types. Sedimentation in sucrose density gradients reveals that large unilamellar vesicles (LUV) and sedimented multilamellar vesicles (sMLV), as opposed to SUV, form lipoplexes that exist as a single phase over a relatively broad range of mixing (+/-) ratios. This is indicated by observing that most of the LUV and sMLV become involved in the assembly reaction up to mixing (+/-) ratios of 4 and 9, respectively, while only a small and constant fraction of SUV associates with DNA at all mixing (+/-) ratios tested. Consequently, while maximal (+/-) ratios of approximately 4.5 and 9 are found in LUV and sMLV lipoplexes, respectively, a final (+/-) ratio of only approximately 2 is determined in SUV lipoplexes. Isothermal titration calorimetry shows that this is the lowest possible charge ratio achieved when liposomes are titrated with DNA. Based on these observations and on the size differences of the liposomes used, a model of lipoplex formation is proposed.
Figures







References
-
- Barron, L. G., L. S. Uyechi, and F. C. Szoka, Jr. 1999. Cationic lipids are essential for gene delivery mediated by intravenous administration of lipoplexes. Gene Ther. 6:1179–1183. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. - PubMed
-
- Boukhnikachvili, T., O. Aguerre-Chariol, M. Airiau, S. Lesieur, M. Ollivon, and J. Vacus. 1997. Structure of in-serum transfecting DNA-cationic lipid complexes. FEBS Lett. 409:188–194. - PubMed
-
- Bruinsma, R. 1998. Electrostatics of DNA-cationic lipid complexes: isoelectric instability. Eur. Phys. J. B. 4:75–88.
-
- deHaseth, P. L., T. M. Lohman, and M. T. Record. 1977. Nonspecific interaction of the lac repressor with DNA: an association reaction driven by counterion release. Biochemistry. 16:4783–4790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

