Elasticity and adhesion force mapping reveals real-time clustering of growth factor receptors and associated changes in local cellular rheological properties
- PMID: 14990502
- PMCID: PMC1304010
- DOI: 10.1016/S0006-3495(04)74243-5
Elasticity and adhesion force mapping reveals real-time clustering of growth factor receptors and associated changes in local cellular rheological properties
Abstract
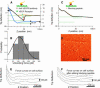
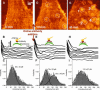
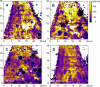
Cell surface macromolecules such as receptors and ion channels serve as the interface link between the cytoplasm and the extracellular region. Their density, distribution, and clustering are key spatial features influencing effective and proper physical and biochemical cellular responses to many regulatory signals. In this study, the effect of plasma-membrane receptor clustering on local cell mechanics was obtained from maps of interaction forces between antibody-conjugated atomic force microscope tips and a specific receptor, a vascular endothelial growth factor (VEGF) receptor. The technique allows simultaneous measurement of the real-time motion of specific macromolecules and their effect on local rheological properties like elasticity. The clustering was stimulated by online additions of VEGF, or antibody against VEGF receptors. VEGF receptors are found to concentrate toward the cell boundaries and cluster rapidly after the online additions commence. Elasticity of regions under the clusters is found to change remarkably, with order-of-magnitude stiffness reductions and fluidity increases. The local stiffness reductions are nearly proportional to receptor density and, being concentrated near the cell edges, provide a mechanism for cell growth and angiogenesis.
Figures

References
-
- Almqvist, N., Y. del Amo, B. L. Smith, N. H. Thomson, Å. Bartholdson, M. Brzenski, R. Lal, and P. K. Hansma. 2001. Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy. J. Microsc. 202:518–532. - PubMed
-
- Antonova, I., O. Arancio, A. C. Trillat, H.-G. Wang, L. Zablow, H. Udo, E. R. Kandel, and R. D. Hawkins. 2001. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 294:1547–1550. - PubMed
-
- Benoit, M., D. Gabriel, G. Gerish, and H. E. Gaub. 2000. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol. 2:313–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

