Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs
- PMID: 14990505
- PMCID: PMC1304013
- DOI: 10.1016/S0006-3495(04)74246-0
Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs
Abstract
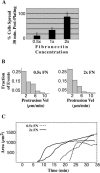
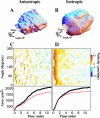
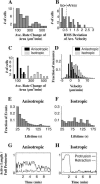
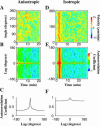
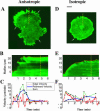
When mouse embryonic fibroblasts in suspension contact a matrix-coated surface, they rapidly adhere and spread. Using total internal reflection fluorescence microscopy of dye-loaded fibroblasts to quantify cell-substrate contact, we found that increasing the surface matrix density resulted in faster spreading initiation whereas lamellipodial dynamics during spreading were unaltered. After spreading initiation, most cells spread in an anisotropic manner through stochastic, transient extension periods (STEPs) with approximately 30 STEPs over 10 min to reach an area of 1300 micro m(2) +/- 300 micro m(2). A second mode of spreading, increased in serum-deprived cells, lacked STEPs and spread in a rapid, isotropic manner for 1-4 min. This isotropic mode was characterized by a high rate of area increase, 340 micro m(2)/min with 78% of the cell edge extending. Anisotropic cells spread slower via STEPs, 126 micro m(2)/min with 34% of the edge extending. During the initial 2-4 min of fast, isotropic spreading, centripetal flow of actin was low (0.8 micro m/min) whereas in anisotropic cells it was high from early times (4.7 micro m/min). After initial isotropic spreading, rearward actin movement increased and isotropic cells displayed STEPs similar to anisotropic cells. Thus, the two cell states display dramatically different spreading whereas long-term motility is based on STEPs.
Figures


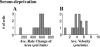

References
-
- Aplin, A. E., B. P. Hogan, J. Tomeu, and R. L. Juliano. 2002. Cell adhesion differentially regulates the nucleocytoplasmic distribution of active MAP kinases. J. Cell Sci. 115:2781–2790. - PubMed
-
- Axelrod, D., N. L. Thompson, and T. P. Burghardt. 1983. Total internal inflection fluorescent microscopy. J. Microsc. 129:19–28. - PubMed
-
- Bailly, M., J. S. Condeelis, and J. E. Segall. 1998. Chemoattractant-induced lamellipod extension. Microsc. Res. Tech. 43:433–443. - PubMed
-
- Bear, J. E., T. M. Svitkina, M. Krause, D. A. Schafer, J. J. Loureiro, G. A. Strasser, I. V. Maly, O. Y. Chaga, J. A. Cooper, G. G. Borisy, and F. B. Gertler. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 109:509–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

