Best Practice No 176: Updated recommendations for HER2 testing in the UK
- PMID: 14990588
- PMCID: PMC1770242
- DOI: 10.1136/jcp.2003.007724
Best Practice No 176: Updated recommendations for HER2 testing in the UK
Abstract
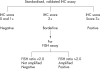
This paper serves to update previously published guidance on rationale and methodology for HER2 laboratory testing following the recommendation for the use of HER2 targeted treatment in the management of advanced breast cancer in the UK. Emphasis is placed on the standardisation of methodology and assessment and strategies to achieve high quality performance. A two phase testing algorithm based on first line immunocytochemistry evaluation and second line fluorescence in situ hybridisation assessment of borderline cases is recommended. To ensure maintenance of expertise, an annual caseload volume of at least 250 cases is recommended for laboratories providing a testing service.
Figures


Comment in
-
Guidelines for HER2 testing in the UK.J Clin Pathol. 2004 Mar;57(3):241-2. doi: 10.1136/jcp.2003.009308. J Clin Pathol. 2004. PMID: 14990591 Free PMC article. No abstract available.
References
-
- Plunkett TA, Miles DW. New biological therapies for breast cancer. Int J Clin Pract 2002;56:261–6. - PubMed
-
- National Institute for Clinical Excellence. Technology Appraisal No. 34. Guidance on the use of trastuzumab for the treatment of advanced breast cancer 2002. (http://www.nice.org.uk/article.asp?a=29280).
-
- Press MF HG, Godolphin W, et al. Sensitivity of HER2/neu antibodies in archival tissue samples: potential source of errors in immunohistochemical studies of oncogene expression. Cancer Res 1994;54:2771–7. - PubMed
-
- Walker R. The significance of histological determination of HER-2 status in breast cancer. Breast 2000;9:130–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
