Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors
- PMID: 14990686
- PMCID: PMC353774
- DOI: 10.1128/jvi.78.6.2666-2673.2004
Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors
Abstract
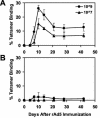
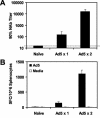
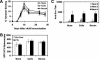
The high prevalence of preexisting immunity to adenovirus serotype 5 (Ad5) in human populations will likely limit the immunogenicity and clinical utility of recombinant Ad5 vector-based vaccines for human immunodeficiency virus type 1 and other pathogens. Ad5-specific neutralizing antibodies (NAbs) are thought to contribute substantially to anti-Ad5 immunity, but the potential importance of Ad5-specific T lymphocytes in this setting has not been fully characterized. Here we assess the relative contributions of Ad5-specific humoral and cellular immune responses in blunting the immunogenicity of a rAd5-Env vaccine in mice. Adoptive transfer of Ad5-specific NAbs resulted in a dramatic abrogation of Env-specific immune responses following immunization with rAd5-Env. Interestingly, adoptive transfer of Ad5-specific CD8(+) T lymphocytes also resulted in a significant and durable suppression of rAd5-Env immunogenicity. These data demonstrate that NAbs and CD8(+) T lymphocytes both contribute to immunity to Ad5. Novel adenovirus vectors that are currently being developed to circumvent the problem of preexisting anti-Ad5 immunity should therefore be designed to evade both humoral and cellular Ad5-specific immune responses.
Figures




References
-
- Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. - PubMed
-
- Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting HIV-1 vaccines. J. Virol. 77:8729-8735. - PMC - PubMed
-
- Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

