Human rhinovirus type 2-antibody complexes enter and infect cells via Fc-gamma receptor IIB1
- PMID: 14990693
- PMCID: PMC353733
- DOI: 10.1128/jvi.78.6.2729-2737.2004
Human rhinovirus type 2-antibody complexes enter and infect cells via Fc-gamma receptor IIB1
Abstract
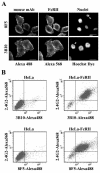
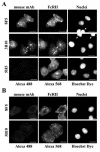
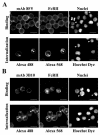
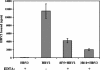
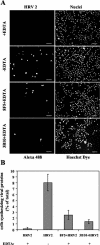
HeLa cells were stably transfected with a cDNA clone encoding the B1 isoform of the mouse FcgammaRII receptor (hereafter referred to as HeLa-FcRII cells). The receptor was expressed at high level at the plasma membrane in about 90% of the cells. These cells bound and internalized mouse monoclonal virus-neutralizing antibodies 8F5 and 3B10 of the subtype immunoglobulin G2a (IgG2a) and IgG1, respectively. Binding of the minor-group human rhinovirus type 2 (HRV2) to its natural receptors, members of the low-density lipoprotein receptor family, is dependent on the presence of Ca(2+) ions. Thus, chelating Ca(2+) ions with EDTA prevented HRV2 binding, entry, and infection. However, upon complex formation of (35)S-labeled HRV2 with 8F5 or 3B10, virus was bound, internalized, and degraded in HeLa-FcRII cells. Furthermore, challenge of these cells with HRV2-8F5 or HRV2-3B10 complexes resulted in de novo synthesis of viral proteins, as shown by indirect immunofluorescence microscopy. These data demonstrate that minor-group receptors can be replaced by surrogate receptors to mediate HRV2 cell entry, delivery into endosomal compartments, and productive uncoating. Consequently, the conformational change and uncoating of HRV2 appears to be solely triggered by the low-pH (pH </= 5.6) environment in these compartments.
Figures


References
-
- Bayer, N., D. Schober, M. Hüttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on HRV2 cell entry. J. Biol. Chem. 276:3952-3962. - PubMed
-
- Brown, M. S., J. Herz, and J. L. Goldstein. 1997. LDL receptor structure: calcium cages, acid baths and recycling receptors. Nature 388:629-630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

