Cul7/p185/p193 binding to simian virus 40 large T antigen has a role in cellular transformation
- PMID: 14990695
- PMCID: PMC353757
- DOI: 10.1128/jvi.78.6.2749-2757.2004
Cul7/p185/p193 binding to simian virus 40 large T antigen has a role in cellular transformation
Abstract
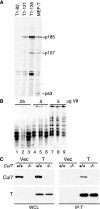
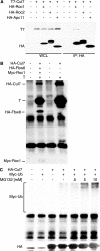
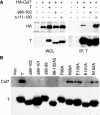
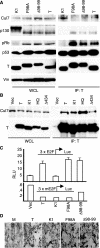
Simian virus 40 large T antigen (TAg) is a viral oncoprotein that can promote cellular transformation. TAg's transforming activity results in part by binding and inactivating key tumor suppressors, including p53 and the retinoblastoma protein (pRb). We have identified a TAg-associated 185-kDa protein that has significant homology to the cullin family of E3 ubiquitin ligases. TAg binds to an SCF-like complex that contains p185/Cul7, Rbx1, and the F box protein Fbw6. This SCF-like complex binds to an N-terminal region of TAg. Several p185/Cul7-binding-deficient mutants of TAg were generated that retained binding to pRb and p53 and were capable of overcoming Rb-mediated repression of E2F transcription. Despite binding to pRb and p53, these p185/Cul7-binding-defective mutants of TAg were unable to transform primary mouse embryo fibroblasts. Cells expressing p185/Cul7-binding-defective mutants of TAg were unable to grow to high density or grow in an anchorage-independent manner as determined by growth in soft agar. Considering the significance of other TAg-interacting proteins in regulation of the cell cycle, p185/Cul7 may also regulate an important growth control pathway.
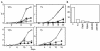
Figures
References
-
- Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 11:15-23. - PubMed
-
- Bai, C., P. Sen, K. Hofmann, L. Ma, M. Goebl, J. W. Harper, and S. J. Elledge. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86:263-274. - PubMed
-
- Bargonetti, J., P. N. Friedman, S. E. Kern, B. Vogelstein, and C. Prives. 1991. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell 65:1083-1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

