Cells expressing the RING finger Z protein are resistant to arenavirus infection
- PMID: 14990716
- PMCID: PMC353761
- DOI: 10.1128/jvi.78.6.2979-2983.2004
Cells expressing the RING finger Z protein are resistant to arenavirus infection
Abstract
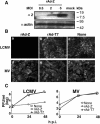
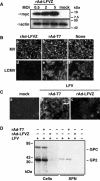
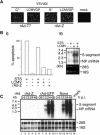
Arenaviruses include Lassa fever virus (LFV) and the South American hemorrhagic fever viruses. These viruses cause severe human disease, and they pose a threat as agents of bioterrorism. Arenaviruses are enveloped viruses with a bisegmented negative-strand RNA genome whose proteomic capability is limited to four polypeptides: nucleoprotein (NP); surface glycoprotein (GP), which is proteolytically processed into GP1 and GP2; polymerase (L); and a small (11-kDa) RING finger protein (Z). Our investigators have previously shown that Z has a strong inhibitory activity on RNA synthesis mediated by the polymerase of the prototypic arenavirus, lymphocytic choriomeningitis virus (LCMV). In this report we show that cells transduced with a replication-deficient recombinant adenovirus expressing Z (rAd-Z) are resistant to LCMV and LFV infection. Virus cell entry mediated by LCMV or LFV GP was not affected in rAd-Z-transduced cells, but both virus transcription and replication were strongly and specifically inhibited, which resulted in a dramatic reduction in production of infectious virus. These findings open new avenues for developing antiviral strategies to combat the highly pathogenic human arenaviruses, including LFV.
Figures
References
-
- Bock, M., M. Heinkelein, D. Lindemann, and A. Rethwilm. 1998. Cells expressing the human foamy virus (HFV) accessory Bet protein are resistant to productive HFV superinfection. Virology 250:194-204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

