Characterization of a neuraminidase-deficient influenza a virus as a potential gene delivery vector and a live vaccine
- PMID: 14990727
- PMCID: PMC353727
- DOI: 10.1128/jvi.78.6.3083-3088.2004
Characterization of a neuraminidase-deficient influenza a virus as a potential gene delivery vector and a live vaccine
Abstract
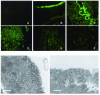
We recently identified a packaging signal in the neuraminidase (NA) viral RNA (vRNA) segment of an influenza A virus, allowing us to produce a mutant virus [GFP(NA)-Flu] that lacks most of the NA open reading frame but contains instead the gene encoding green fluorescent protein (GFP). To exploit the expanding knowledge of vRNA packaging signals to establish influenza virus vectors for the expression of foreign genes, we studied the replicative properties of this virus in cell culture and mice. Compared to wild-type virus, GFP(NA)-Flu was highly attenuated in normal cultured cells but was able to grow to a titer of >10(6) PFU/ml in a mutant cell line expressing reduced levels of sialic acid on the cell surface. GFP expression from this virus was stable even after five passages in the latter cells. In intranasally infected mice, GFP was detected in the epithelial cells of nasal mucosa, bronchioles, and alveoli for up to 4 days postinfection. We attribute the attenuated growth of GFP(NA)-Flu to virion aggregation at the surface of bronchiolar epithelia. In studies to test the potential of this mutant as a live attenuated influenza vaccine, all mice vaccinated with >/==" BORDER="0">10(5) PFU of GFP(NA)-Flu survived when challenged with lethal doses of the parent virus. These results suggest that influenza virus could be a useful vector for expressing foreign genes and that a sialidase-deficient virus may offer an alternative to the live influenza vaccines recently approved for human use.
Figures

References
-
- Boivin, G., N. Goyette, and H. Bernatchez. 2002. Prolonged excretion of amantadine-resistant influenza A virus quasispecies after cessation of antiviral therapy in an immunocompromised patient. Clin. Infect. Dis. 34:e23-e25. - PubMed
-
- Bramson, J. L., and Y. H. Wan. 2002. The efficacy of genetic vaccination is dependent upon the nature of the vector system and antigen. Expert Opin. Biol. Ther. 2:75-85. - PubMed
-
- Capua, I., C. Terregino, G. Cattoli, F. Mutinelli, and J. F. Rodriguez. 2003. Development of a DIVA (differentiating infected from vaccinated animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol. 32:47-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

