5'-untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation
- PMID: 14990743
- PMCID: PMC390293
- DOI: 10.1093/nar/gkh305
5'-untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation
Abstract
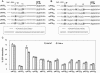
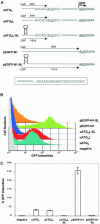
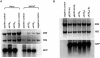
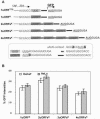
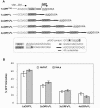
Upstream AUGs (uAUGs) and upstream open reading frames (uORFs) are common features of mRNAs that encode regulatory proteins and have been shown to profoundly influence translation of the main ORF. In this study, we employed a series of artificial 5'-untranslated regions (5'-UTRs) containing one or more uAUGs/uORFs to systematically assess translation initiation at the main AUG by leaky scanning and reinitiation mechanisms. Constructs containing either one or two uAUGs in varying contexts but without an in-frame stop codon upstream of the main AUG were used to analyse the leaky scanning mechanism. This analysis largely confirmed the ranking of different AUG contextual sequences that was determined previously by Kozak. In addition, this ranking was the same for both the first and second uAUGs, although the magnitude of initiation efficiency differed. Moreover, approximately 10% of ribosomes exhibited leaky scanning at uAUGs in the most favourable context and initiated at a downstream AUG. A second group of constructs containing different numbers of uORFs, each with optimal uAUGs, were used to measure the capacity for reinitiation. We found significant levels of initiation at the main ORF even in constructs containing four uORFs, with nearly 10% of ribosomes capable of reinitiating five times. This study shows that for mRNAs containing multiple uORFs/uAUGs, ribosome reinitiation and leaky scanning are efficient mechanisms for initiation at their main AUGs.
Figures

References
-
- Hellen C.U. and Sarnow,P. (2001) Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev., 15, 1593–1612. - PubMed
-
- Hershey J. and Merrick,W. (2000) The pathway and mechanism of initiation of protein synthesis. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 38–88.
-
- Cazzola M. and Skoda,R. (2000) Translational pathophysiology: a novel molecular mechanism of human disease. Blood, 95, 3280–3288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

