The fibrinolytic system attenuates vascular tone: effects of tissue plasminogen activator (tPA) and aminocaproic acid on renal microcirculation
- PMID: 14993107
- PMCID: PMC1574281
- DOI: 10.1038/sj.bjp.0705714
The fibrinolytic system attenuates vascular tone: effects of tissue plasminogen activator (tPA) and aminocaproic acid on renal microcirculation
Abstract
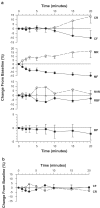
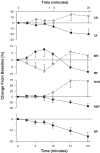
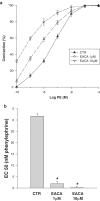
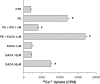
1. The renal medulla is a major source of plasminogen activators (PA), recently shown to induce vasodilation in vitro. Treatment with PA inhibitors has been associated with renal dysfunction, suggesting compromised renal microvasculature. We investigated the impact of the PA inhibitor epsilon amino-caproic acid (EACA) upon vascular tone in vitro, and studied the effect of both tPA and EACA upon intrarenal hemodynamics in vivo. 2. In vitro experiments were carried out in isolated aortic rings and with cultured vascular smooth muscle cells. Studies of renal microcirculation and morphology were conducted in anesthetized Sprague-Dawley rats. 3. In isolated aortic rings, EACA (but not the other inhibitors of the fibrinolytic system PAI-1 or alpha-2 antiplasmin) reduced the half-maximal effective concentration of phenylephrine (PE) required to induce contraction (from 32 nm in control solution to 2 and 0.1 nm at EACA concentrations of 1 and 10 microm, respectively). Using reteplase (retavase) in the same model, we also provide evidence that the vasoactivity of tPA is in part kringle-dependent. In cultured vascular smooth muscle cells, Ca(2+) internalization following PE was enhanced by EACA, and retarded by tPA. 4. In anesthetized rats, EACA (150 mg x kg(-1)) did not affect systemic blood pressure, total renal or cortical blood flow. However, the outer medullary blood flow declined 12+/-2% below the baseline (P<0.03). By contrast, tPA (2 mg x kg(-1)), transiently increased outer medullary blood flow by 8+/-5% (P<0.02). Fibrin microthrombi were not found within the renal microvasculature in EACA-treated animals. 5. In conclusion, both fibrinolytic and antifibrinolytic agents modulate medullary renal blood flow with reciprocal effects of vasodilation (PA) and vasoconstriction (EACA). In vitro studies suggest that these hemodynamic responses are related to direct modulation of the vascular tone.
Figures

Similar articles
-
Comparison of recombinant plasminogen activator inhibitor-1 and epsilon amino caproic acid in a hemorrhagic rabbit model.Thromb Haemost. 1992 Jun 1;67(6):692-6. Thromb Haemost. 1992. PMID: 1509411
-
In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone.Blood. 2004 Feb 1;103(3):897-902. doi: 10.1182/blood-2003-05-1685. Epub 2003 Sep 25. Blood. 2004. PMID: 14512309
-
Comparison of the vasodilatory effects of bradykinin in isolated dog renal arteries and in buffer-perfused dog kidneys.Acta Physiol Hung. 1996;84(1):9-18. Acta Physiol Hung. 1996. PMID: 8993670
-
Fibrinolysis in patients with acute ischaemic heart disease. With particular reference to systemic effects of tissue-type plasminogen activator treatment on fibrinolysis, coagulation and complement pathways.Dan Med Bull. 1993 Sep;40(4):383-408. Dan Med Bull. 1993. PMID: 8222763 Review.
-
Direct studies on the control of the renal microcirculation.J Am Soc Nephrol. 1991 Aug;2(2):136-49. doi: 10.1681/ASN.V22136. J Am Soc Nephrol. 1991. PMID: 1954326 Review.
Cited by
-
The Role of Fibrinolytic Regulators in Vascular Dysfunction of Systemic Sclerosis.Int J Mol Sci. 2019 Jan 31;20(3):619. doi: 10.3390/ijms20030619. Int J Mol Sci. 2019. PMID: 30709025 Free PMC article. Review.
-
Incidence and risk factors of contrast-induced nephropathy in acute stroke patients undergoing computed tomography angiography: A single-center study.Neurosciences (Riyadh). 2023 Oct;28(4):258-263. doi: 10.17712/nsj.2023.4.20230030. Neurosciences (Riyadh). 2023. PMID: 37844941 Free PMC article.
-
Therapeutic targeting of extracellular DNA improves the outcome of intestinal ischemic reperfusion injury in neonatal rats.Sci Rep. 2017 Nov 13;7(1):15377. doi: 10.1038/s41598-017-15807-6. Sci Rep. 2017. PMID: 29133856 Free PMC article.
-
Oligonucleotide Microarrays Identified Potential Regulatory Genes Related to Early Outward Arterial Remodeling Induced by Tissue Plasminogen Activator.Front Physiol. 2019 Apr 30;10:493. doi: 10.3389/fphys.2019.00493. eCollection 2019. Front Physiol. 2019. PMID: 31114508 Free PMC article.
-
Plasminogen activator inhibitor-1 regulates myoendothelial junction formation.Circ Res. 2010 Apr 2;106(6):1092-102. doi: 10.1161/CIRCRESAHA.109.215723. Epub 2010 Feb 4. Circ Res. 2010. PMID: 20133900 Free PMC article.
References
-
- ANDERSSON L.G., EKROTH R., BRATTEBY L.E. Acute renal failure after coronary surgery – a study of incidence and risk factors in 2009 consecutive patients. Thorac. Cardiovasc. Surg. 1993;41:237–241. - PubMed
-
- BIESSEN E.A., VAN TEIJLINGEN M., VIETSCH H., BARRETT-BERGSHOEFF M.M., BIJSTERBOSCH M.K., RIJKEN D.C., VAN BERKEL T.J., KUIPER J. Antagonists of the mannose receptor and the LDL receptor-related protein dramatically delay the clearance of tissue plasminogen activator. Circulation. 1997;95:46–52. - PubMed
-
- BREZIS M., HEYMAN S.N., EPSTEIN F. Determinants of intrarenal oxygenation: 2. Hemodynamic effects. Am. J. Physiol. 1994;267:F1063–F1068. - PubMed
-
- BREZIS M., ROSEN S. Hypoxia of the renal medulla – its implications for disease. N. Engl. J. Med. 1995;332:647–655. - PubMed
-
- CHARYTAN C., PURTILO D. Glomerular capillary thrombosis and acute renal failure after epsilon-amino caproic acid therapy. N. Engl. J. Med. 1969;280:1102–1104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

