Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin
- PMID: 14993232
- PMCID: PMC2172172
- DOI: 10.1083/jcb.200307032
Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin
Abstract
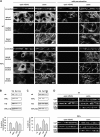
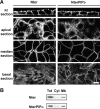
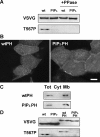
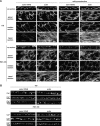
Ezrin, a membrane-actin cytoskeleton linker, which participates in epithelial cell morphogenesis, is held inactive in the cytoplasm through an intramolecular interaction. Phosphatidylinositol 4,5-bisphosphate (PIP2) binding and the phosphorylation of threonine 567 (T567) are involved in the activation process that unmasks both membrane and actin binding sites. Here, we demonstrate that ezrin binding to PIP2, through its NH2-terminal domain, is required for T567 phosphorylation and thus for the conformational activation of ezrin in vivo. Furthermore, we found that the T567D mutation mimicking T567 phosphorylation bypasses the need for PIP2 binding for unmasking both membrane and actin binding sites. However, PIP2 binding and T567 phosphorylation are both necessary for the correct apical localization of ezrin and for its role in epithelial cell morphogenesis. These results establish that PIP2 binding and T567 phosphorylation act sequentially to allow ezrin to exert its cellular functions.
Figures

References
-
- Bretscher, A., K. Edwards, and R.G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3:586–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

