Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4
- PMID: 14993234
- PMCID: PMC2172161
- DOI: 10.1083/jcb.200312070
Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4
Abstract
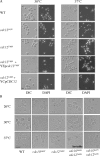
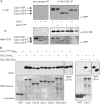
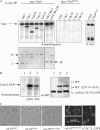
Assembly at the mother-bud neck of a filamentous collar containing five septins (Cdc3, Cdc10, Cdc11, Cdc12, and Shs1) is necessary for proper morphogenesis and cytokinesis. We show that Cdc10 and Cdc12 possess GTPase activity and appropriate mutations in conserved nucleotide-binding residues abrogate GTP binding and/or hydrolysis in vitro. In vivo, mutants unable to bind GTP prevent septin collar formation, whereas mutants that block GTP hydrolysis do not. GTP binding-defective Cdc10 and Cdc12 form soluble heteromeric complexes with other septins both in yeast and in bacteria; yet, unlike wild-type, mutant complexes do not bind GTP and do not assemble into filaments in vitro. Absence of a p21-activated protein kinase (Cla4) perturbs septin collar formation. This defect is greatly exacerbated when combined with GTP binding-defective septins; conversely, the septin collar assembly defect of such mutants is suppressed efficiently by CLA4 overexpression. Cla4 interacts directly with and phosphorylates certain septins in vitro and in vivo. Thus, septin collar formation may correspond to septin filament assembly, and requires both GTP binding and Cla4-mediated phosphorylation of septins.
Figures





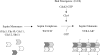
Similar articles
-
Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae.Mol Biol Cell. 2004 Oct;15(10):4568-83. doi: 10.1091/mbc.e04-04-0330. Epub 2004 Jul 28. Mol Biol Cell. 2004. PMID: 15282341 Free PMC article.
-
Higher-order septin assembly is driven by GTP-promoted conformational changes: evidence from unbiased mutational analysis in Saccharomyces cerevisiae.Genetics. 2014 Mar;196(3):711-27. doi: 10.1534/genetics.114.161182. Epub 2014 Jan 7. Genetics. 2014. PMID: 24398420 Free PMC article.
-
Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae.Mol Biol Cell. 2004 Dec;15(12):5329-45. doi: 10.1091/mbc.e04-03-0254. Epub 2004 Sep 15. Mol Biol Cell. 2004. PMID: 15371547 Free PMC article.
-
Septin mutations and phenotypes in S. cerevisiae.Cytoskeleton (Hoboken). 2019 Jan;76(1):33-44. doi: 10.1002/cm.21492. Epub 2018 Nov 18. Cytoskeleton (Hoboken). 2019. PMID: 30171672 Review.
-
Diversity of septin scaffolds.Curr Opin Cell Biol. 2006 Feb;18(1):54-60. doi: 10.1016/j.ceb.2005.12.005. Epub 2005 Dec 13. Curr Opin Cell Biol. 2006. PMID: 16356703 Review.
Cited by
-
Regulation of phosphoinositide levels by the phospholipid transfer protein Sec14p controls Cdc42p/p21-activated kinase-mediated cell cycle progression at cytokinesis.Eukaryot Cell. 2007 Oct;6(10):1814-23. doi: 10.1128/EC.00087-07. Epub 2007 Jun 29. Eukaryot Cell. 2007. PMID: 17601877 Free PMC article.
-
Vhs2 is a novel regulator of septin dynamics in budding yeast.Cell Cycle. 2014;13(10):1590-601. doi: 10.4161/cc.28561. Epub 2014 Mar 19. Cell Cycle. 2014. PMID: 24646733 Free PMC article.
-
PakD, a putative p21-activated protein kinase in Dictyostelium discoideum, regulates actin.Eukaryot Cell. 2014 Jan;13(1):119-26. doi: 10.1128/EC.00216-13. Epub 2013 Nov 15. Eukaryot Cell. 2014. PMID: 24243792 Free PMC article.
-
Regulation of distinct septin rings in a single cell by Elm1p and Gin4p kinases.Mol Biol Cell. 2009 Apr;20(8):2311-26. doi: 10.1091/mbc.e08-12-1169. Epub 2009 Feb 18. Mol Biol Cell. 2009. PMID: 19225152 Free PMC article.
-
A lysine deacetylase Hos3 is targeted to the bud neck and involved in the spindle position checkpoint.Mol Biol Cell. 2014 Sep 15;25(18):2720-34. doi: 10.1091/mbc.E13-10-0619. Epub 2014 Jul 23. Mol Biol Cell. 2014. PMID: 25057019 Free PMC article.
References
-
- Barral, Y., V. Mermali, M.S. Mooseker, and M. Snyder. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell. 5:841–851. - PubMed
-
- Boeke, J.D., J. Trueheart, G. Natsoulis, and G.R. Fink. 1987. 5-Flouroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164–175. - PubMed
-
- Bouquin, N., Y. Barral, R. Courbeyrette, M. Blondel, M. Snyder, and C. Mann. 2000. Regulation of cytokinesis by the Elm1 protein kinase in Saccharomyces cerevisiae. J. Cell Sci. 113:1435–1445. - PubMed

