Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate
- PMID: 14993235
- PMCID: PMC2172159
- DOI: 10.1083/jcb.200312045
Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate
Abstract
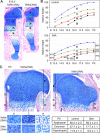
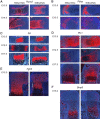
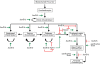
Sox5 and Sox6 encode Sry-related transcription factors that redundantly promote early chondroblast differentiation. Using mouse embryos with three or four null alleles of Sox5 and Sox6, we show that they are also essential and redundant in major steps of growth plate chondrocyte differentiation. Sox5 and Sox6 promote the development of a highly proliferating pool of chondroblasts between the epiphyses and metaphyses of future long bones. This pool is the likely cellular source of growth plates. Sox5 and Sox6 permit formation of growth plate columnar zones by keeping chondroblasts proliferating and by delaying chondrocyte prehypertrophy. They allow induction of chondrocyte hypertrophy and permit formation of prehypertrophic and hypertrophic zones by delaying chondrocyte terminal differentiation induced by ossification fronts. They act, at least in part, by down-regulating Ihh signaling, Fgfr3, and Runx2 and by up-regulating Bmp6. In conclusion, Sox5 and Sox6 are needed for the establishment of multilayered growth plates, and thereby for proper and timely development of endochondral bones.
Figures






Similar articles
-
The transcription factors L-Sox5 and Sox6 are essential for cartilage formation.Dev Cell. 2001 Aug;1(2):277-90. doi: 10.1016/s1534-5807(01)00003-x. Dev Cell. 2001. PMID: 11702786
-
Targeted expression of SHH affects chondrocyte differentiation, growth plate organization, and Sox9 expression.J Bone Miner Res. 2004 Oct;19(10):1678-88. doi: 10.1359/JBMR.040706. Epub 2004 Jul 12. J Bone Miner Res. 2004. PMID: 15355563
-
Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs.Development. 2003 Mar;130(6):1135-48. doi: 10.1242/dev.00331. Development. 2003. PMID: 12571105
-
Interaction of growth factors regulating chondrocyte differentiation in the developing embryo.Osteoarthritis Cartilage. 2001;9 Suppl A:S109-17. Osteoarthritis Cartilage. 2001. PMID: 11680674 Review.
-
Toward understanding the functions of the two highly related Sox5 and Sox6 genes.J Bone Miner Metab. 2002;20(3):121-30. doi: 10.1007/s007740200017. J Bone Miner Metab. 2002. PMID: 11984693 Review. No abstract available.
Cited by
-
SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells.Stem Cell Res Ther. 2012;3(3):22. doi: 10.1186/scrt113. Stem Cell Res Ther. 2012. PMID: 22742415 Free PMC article.
-
Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development.J Cell Biol. 2007 May 7;177(3):451-64. doi: 10.1083/jcb.200612023. Epub 2007 Apr 30. J Cell Biol. 2007. PMID: 17470636 Free PMC article.
-
Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription.Mol Biol Cell. 2011 Apr 15;22(8):1300-11. doi: 10.1091/mbc.E10-07-0566. Epub 2011 Feb 23. Mol Biol Cell. 2011. PMID: 21346191 Free PMC article.
-
Regulating the fate of stem cells for regenerating the intervertebral disc degeneration.World J Stem Cells. 2021 Dec 26;13(12):1881-1904. doi: 10.4252/wjsc.v13.i12.1881. World J Stem Cells. 2021. PMID: 35069988 Free PMC article. Review.
-
Transcriptional control of mesenchymal stem cell differentiation.Transfus Med Hemother. 2008 Jun;35(3):216-27. doi: 10.1159/000127448s. Epub 2008 May 8. Transfus Med Hemother. 2008. PMID: 21547119 Free PMC article. Review.
References
-
- Abad, V., J.L. Meyers, M. Weise, R.I. Gafni, K.M. Barnes, O. Nilsson, J.D. Bacher, and J. Baron. 2002. The role of the resting zone in growth plate chondrogenesis. Endocrinology. 143:1851–1857. - PubMed
-
- Baur, S.T., J.J. Mai, and S.M. Dymecki. 2000. Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity. Development. 127:605–619. - PubMed
-
- Bi, W., J.M. Deng, Z. Zhang, R.R. Behringer, and B. de Crombrugghe. 1999. Sox9 is required for cartilage formation. Nat. Genet. 22:85–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

