LQT4 gene: the "missing" ankyrin
- PMID: 14993420
- PMCID: PMC1618879
- DOI: 10.1124/mi.3.3.131
LQT4 gene: the "missing" ankyrin
Abstract
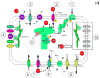
Mutations in ion channels have been implicated in the formation of long QT syndrome (LQTS). However, Mohler et al. have recently uncovered a role for ankyrin-B, a non-ion channel protein, in type IV LQTS. Calcium signalling is altered, and the functions of several channels and pumps that normally interact with wild-type ankyrin-B are impaired in the presence of mutant ankyrin-B. The authors suggest that by disrupting the functions of these channels, a new mechanism has been uncovered that can lead to cardiac myopathy.
Figures
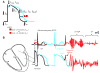


References
-
- Wang Q, Chen Q, Towbin JA. Genetics, molecular mechanisms and management of long QT syndrome. Ann Med. 1998;30:58–65. - PubMed
-
- Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995;4:1603–1607. - PubMed
-
- Vincent GM. The molecular genetics of the long QT syndrome: Genes causing fainting and sudden death. Annu Rev Med. 1998;49:263–274. - PubMed
-
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT.A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome Cell 80795–803.1995A key publication on identification of a cardiac potassium channel a-subunit geneHERG(KCNH2) as the chromosome 7q35-36-linked LQTS gene (LQT2). - PubMed
-
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT.SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome Cell 80805–811.1995A key publication on identification of the cardiac sodium channel geneSCN5Aas the chromosome 3p21-linked LQTS gene (LQT3). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
