Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells
- PMID: 14993597
- PMCID: PMC373505
- DOI: 10.1073/pnas.0400373101
Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells
Abstract
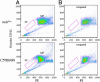
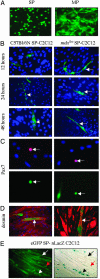
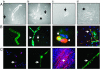
Cell-based therapy for Duchenne muscular dystrophy patients and mdx mice has proven to be a safe but ineffective form of treatment. Recently, a group of cells called muscle side population (SP) cells have been isolated based on their ability to efflux the DNA-binding dye Hoechst. To understand the potential of skeletal muscle SP cells to serve as precursors for muscle, SP cells from the two mice strains mdx(5cv) and C57BL/6N were isolated, transduced, and transplanted. Under coculture conditions with myogenic cells, some cells within the SP cell population can give rise to early Pax7-positive satellite cells and other later stage myogenic cells. Transduced SP cells were transplanted via the tail vein and were shown to successfully deliver enhanced GFP and human microdystrophin to the skeletal muscle of nonirradiated mdx(5cv) mice, thus demonstrating their ability to travel through the capillaries and enter into damaged muscle. These results demonstrate that i.v. delivery of genes via SP cells is possible and that these SP cells are capable of recapitulating the myogenic lineage. Because this approach shows definitive engraftment by using autologous transplantation of noninjured recipients, our data may have substantial implications for therapy of muscular dystrophy.
Figures

References
-
- Hoffman, E. P., Brown, R. H., Jr., & Kunkel, L. M. (1987) Cell 51, 919-928. - PubMed
-
- Cox, G. A., Cole, N. M., Matsumura, K., Phelps, S. F., Hauschka, S. D., Campbell, K. P., Faulkner, J. A. & Chamberlain, J. S. (1993) Nature 364, 725-729. - PubMed
-
- Tremblay, J. P., Malouin, F., Roy, R., Huard, J., Bouchard, J. P., Satoh, A. & Richards, C. L. (1993) Cell Transplant. 2, 99-112. - PubMed
-
- Mendell, J. R., Kissel, J. T., Amato, A. A., King, W., Signore, L., Prior, T. W., Sahenk, Z., Benson, S., McAndrew, P. E., Rice, R., et al. (1995) N. Engl. J. Med. 333, 832-838. - PubMed
-
- Gussoni, E., Blau, H. M. & Kunkel, L. M. (1997) Nat. Med. 3, 970-977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

