Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex
- PMID: 14996805
- PMCID: PMC355973
- DOI: 10.1128/JB.186.6.1737-1746.2003
Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex
Abstract
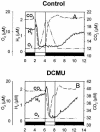
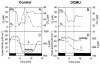
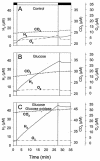
The interaction between hydrogen metabolism, respiration, and photosynthesis was studied in vivo in whole cells of Synechocystis sp. strain PCC 6803 by continuously monitoring the changes in gas concentrations (H2, CO2, and O2) with an online mass spectrometer. The in vivo activity of the bidirectional [NiFe]hydrogenase [H2:NAD(P) oxidoreductase], encoded by the hoxEFUYH genes, was also measured independently by the proton-deuterium (H-D) exchange reaction in the presence of D2. This technique allowed us to demonstrate that the hydrogenase was insensitive to light, was reversibly inactivated by O2, and could be quickly reactivated by NADH or NADPH (+H2). H2 was evolved by cells incubated anaerobically in the dark, after an adaptation period. This dark H2 evolution was enhanced by exogenously added glucose and resulted from the oxidation of NAD(P)H produced by fermentation reactions. Upon illumination, a short (less than 30-s) burst of H2 output was observed, followed by rapid H2 uptake and a concomitant decrease in CO2 concentration in the cyanobacterial cell suspension. Uptake of both H2 and CO2 was linked to photosynthetic electron transport in the thylakoids. In the ndhB mutant M55, which is defective in the type I NADPH-dehydrogenase complex (NDH-1) and produces only low amounts of O2 in the light, H2 uptake was negligible during dark-to-light transitions, allowing several minutes of continuous H2 production. A sustained rate of photoevolution of H2 corresponding to 6 micro mol of H2 mg of chlorophyll(-1) h(-1) or 2 ml of H2 liter(-1) h(-1) was observed over a longer time period in the presence of glucose and was slightly enhanced by the addition of the O2 scavenger glucose oxidase. By the use of the inhibitors DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea] and DBMIB (2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone), it was shown that two pathways of electron supply for H2 production operate in M55, namely photolysis of water at the level of photosystem II and carbohydrate-mediated reduction of the plastoquinone pool.
Figures





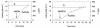
References
-
- Abdel-Basset, R., and K. P. Bader. 1998. Physiological analyses of the hydrogen gas exchange in cyanobacteria. J. Photochem. Photobiol. B Biol. 43:146-151.
-
- Appel, J., S. Phunpruch, K. Steinmuller, and R. Schulz. 2000. The bidirectional hydrogenase of Synechocystis sp PCC 6803 works as an electron valve during photosynthesis. Arch. Microbiol. 173:333-338. - PubMed
-
- Appel, J., and R. Schulz. 1998. Hydrogen metabolism in organisms with oxygenic photosynthesis: hydrogenases as important regulatory devices for a proper redox poising? J. Photochem. Photobiol. B Biol. 47:1-11.
-
- Appel, J., and R. Schulz. 1996. Sequence analysis of an operon of a NAD(P)-reducing nickel hydrogenase from the cyanobacterium Synechocystis sp PCC 6803 gives additional evidence for direct coupling of the enzyme to NAD(P)H-dehydrogenase (complex I). Biochim. Biophys. Acta 1298:141-147. - PubMed
-
- Boison, G., H. Bothe, A. Hansel, and P. Lindblad. 1999. Evidence against a common use of the diaphorase subunits by the bidirectional hydrogenase and by the respiratory complex I in cyanobacteria. FEMS Microbiol. Lett. 174:159-165.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

