Quorum sensing in Staphylococcus aureus biofilms
- PMID: 14996815
- PMCID: PMC355980
- DOI: 10.1128/JB.186.6.1838-1850.2004
Quorum sensing in Staphylococcus aureus biofilms
Abstract
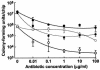
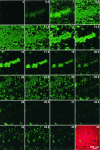
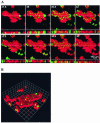
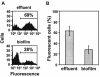
Several serious diseases are caused by biofilm-associated Staphylococcus aureus, infections in which the accessory gene regulator (agr) quorum-sensing system is thought to play an important role. We studied the contribution of agr to biofilm development, and we examined agr-dependent transcription in biofilms. Under some conditions, disruption of agr expression had no discernible influence on biofilm formation, while under others it either inhibited or enhanced biofilm formation. Under those conditions where agr expression enhanced biofilm formation, biofilms of an agr signaling mutant were particularly sensitive to rifampin but not to oxacillin. Time lapse confocal scanning laser microscopy showed that, similar to the expression of an agr-independent fluorescent reporter, biofilm expression of an agr-dependent reporter was in patches within cell clusters and oscillated with time. In some cases, loss of fluorescence appeared to coincide with detachment of cells from the biofilm. Our studies indicate that the role of agr expression in biofilm development and behavior depends on environmental conditions. We also suggest that detachment of cells expressing agr from biofilms may have important clinical implications.
Figures



Similar articles
-
Agr-mediated dispersal of Staphylococcus aureus biofilms.PLoS Pathog. 2008 Apr 25;4(4):e1000052. doi: 10.1371/journal.ppat.1000052. PLoS Pathog. 2008. PMID: 18437240 Free PMC article.
-
Generation of virulence factor variants in Staphylococcus aureus biofilms.J Bacteriol. 2007 Nov;189(22):7961-7. doi: 10.1128/JB.00789-07. Epub 2007 Aug 3. J Bacteriol. 2007. PMID: 17675387 Free PMC article.
-
Biofilm formation and dispersal of Staphylococcus aureus under the influence of oxacillin.Microb Pathog. 2013 Aug-Sep;61-62:66-72. doi: 10.1016/j.micpath.2013.05.002. Epub 2013 May 25. Microb Pathog. 2013. PMID: 23711963
-
Quorum sensing-mediated regulation of staphylococcal virulence and antibiotic resistance.Future Microbiol. 2014;9(5):669-81. doi: 10.2217/fmb.14.31. Future Microbiol. 2014. PMID: 24957093 Review.
-
What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia?Trends Microbiol. 2014 Dec;22(12):676-85. doi: 10.1016/j.tim.2014.09.002. Epub 2014 Oct 6. Trends Microbiol. 2014. PMID: 25300477 Review.
Cited by
-
The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response.Microbiol Spectr. 2016 Apr;4(2):10.1128/microbiolspec.VMBF-0022-2015. doi: 10.1128/microbiolspec.VMBF-0022-2015. Microbiol Spectr. 2016. PMID: 27227309 Free PMC article. Review.
-
Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal.Nat Rev Microbiol. 2011 Nov 28;10(1):39-50. doi: 10.1038/nrmicro2695. Nat Rev Microbiol. 2011. PMID: 22120588 Review.
-
Coral-Associated Bacteria as a Promising Antibiofilm Agent against Methicillin-Resistant and -Susceptible Staphylococcus aureus Biofilms.Evid Based Complement Alternat Med. 2012;2012:862374. doi: 10.1155/2012/862374. Epub 2012 Sep 4. Evid Based Complement Alternat Med. 2012. PMID: 22988476 Free PMC article.
-
Biofilms.Cold Spring Harb Perspect Biol. 2010 Jul;2(7):a000398. doi: 10.1101/cshperspect.a000398. Epub 2010 Jun 2. Cold Spring Harb Perspect Biol. 2010. PMID: 20519345 Free PMC article. Review.
-
Molecular characterization of a novel Staphylococcus aureus surface protein (SasC) involved in cell aggregation and biofilm accumulation.PLoS One. 2009 Oct 23;4(10):e7567. doi: 10.1371/journal.pone.0007567. PLoS One. 2009. PMID: 19851500 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

