Structural characterization of the SARS-coronavirus spike S fusion protein core
- PMID: 14996844
- PMCID: PMC8060857
- DOI: 10.1074/jbc.M400759200
Structural characterization of the SARS-coronavirus spike S fusion protein core
Abstract
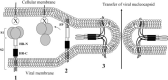
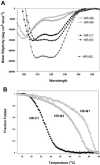
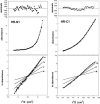
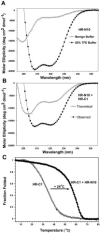
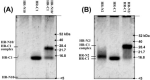
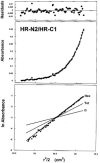
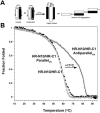
The spike (S) glycoprotein of coronaviruses mediates viral entry into host cells. It is a type 1 viral fusion protein that characteristically contains two heptad repeat regions, denoted HR-N and HR-C, that form coiled-coil structures within the ectodomain of the protein. Previous studies have shown that the two heptad repeat regions can undergo a conformational change from their native state to a 6-helix bundle (trimer of dimers), which mediates fusion of viral and host cell membranes. Here we describe the biophysical analysis of the two predicted heptad repeat regions within the severe acute respiratory syndrome coronavirus S protein. Our results show that in isolation the HR-N region forms a stable alpha-helical coiled coil that associates in a tetrameric state. The HR-C region in isolation formed a weakly stable trimeric coiled coil. When mixed together, the two peptide regions (HR-N and HR-C) associated to form a very stable alpha-helical 6-stranded structure (trimer of heterodimers). Systematic peptide mapping showed that the site of interaction between the HR-N and HR-C regions is between residues 916-950 of HR-N and residues 1151-1185 of HR-C. Additionally, interchain disulfide bridge experiments showed that the relative orientation of the HR-N and HR-C helices in the complex was antiparallel. Overall, the structure of the hetero-stranded complex is consistent with the structures observed for other type 1 viral fusion proteins in their fusion-competent state.
Figures



Similar articles
-
Template-based coiled-coil antigens elicit neutralizing antibodies to the SARS-coronavirus.J Struct Biol. 2006 Aug;155(2):176-94. doi: 10.1016/j.jsb.2006.03.019. Epub 2006 Apr 27. J Struct Biol. 2006. PMID: 16697221 Free PMC article.
-
Characterization of the heptad repeat regions, HR1 and HR2, and design of a fusion core structure model of the spike protein from severe acute respiratory syndrome (SARS) coronavirus.Biochemistry. 2004 Nov 9;43(44):14064-71. doi: 10.1021/bi049101q. Biochemistry. 2004. PMID: 15518555
-
Biophysical characterization of HRC peptide analogs interaction with heptad repeat regions of the SARS-coronavirus Spike fusion protein core.J Struct Biol. 2006 Aug;155(2):162-75. doi: 10.1016/j.jsb.2006.03.024. Epub 2006 Apr 27. J Struct Biol. 2006. PMID: 16765058 Free PMC article.
-
Solution structure of the severe acute respiratory syndrome-coronavirus heptad repeat 2 domain in the prefusion state.J Biol Chem. 2006 Apr 28;281(17):11965-71. doi: 10.1074/jbc.M601174200. Epub 2006 Feb 28. J Biol Chem. 2006. PMID: 16507566 Free PMC article.
-
The SARS-CoV S glycoprotein.Cell Mol Life Sci. 2004 Oct;61(19-20):2428-30. doi: 10.1007/s00018-004-4257-y. Cell Mol Life Sci. 2004. PMID: 15526150 Free PMC article. Review.
Cited by
-
SARS-COV-2 (COVID-19): Cellular and biochemical properties and pharmacological insights into new therapeutic developments.Cell Biochem Funct. 2021 Jan;39(1):10-28. doi: 10.1002/cbf.3591. Epub 2020 Sep 29. Cell Biochem Funct. 2021. PMID: 32992409 Free PMC article. Review.
-
Template-based coiled-coil antigens elicit neutralizing antibodies to the SARS-coronavirus.J Struct Biol. 2006 Aug;155(2):176-94. doi: 10.1016/j.jsb.2006.03.019. Epub 2006 Apr 27. J Struct Biol. 2006. PMID: 16697221 Free PMC article.
-
Genetic analysis of the SARS-coronavirus spike glycoprotein functional domains involved in cell-surface expression and cell-to-cell fusion.Virology. 2005 Oct 25;341(2):215-30. doi: 10.1016/j.virol.2005.06.046. Epub 2005 Aug 15. Virology. 2005. PMID: 16099010 Free PMC article.
-
Design and biological activities of novel inhibitory peptides for SARS-CoV spike protein and angiotensin-converting enzyme 2 interaction.Antiviral Res. 2006 Feb;69(2):70-6. doi: 10.1016/j.antiviral.2005.10.005. Epub 2005 Nov 28. Antiviral Res. 2006. PMID: 16337697 Free PMC article.
-
Structural characterization of the fusion-active complex of severe acute respiratory syndrome (SARS) coronavirus.Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8709-14. doi: 10.1073/pnas.0402753101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161975 Free PMC article.
References
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

