Phase II study of neoadjuvant paclitaxel and cisplatin for operable and locally advanced breast cancer: analysis of 126 patients
- PMID: 14997191
- PMCID: PMC2409621
- DOI: 10.1038/sj.bjc.6601616
Phase II study of neoadjuvant paclitaxel and cisplatin for operable and locally advanced breast cancer: analysis of 126 patients
Abstract
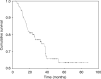
In an earlier study, we have demonstrated a high clinical and pathologic response rate of neoadjuvant paclitaxel (P) and cisplatin (C) for patients with locally advanced breast cancer (LABC). The current phase II study includes larger number of patients who had longer follow-up. A total of 126 consecutive patients with noninflammatory LABC (T2 >4 cm, T3 or T4, N0-N3, M0) were included in the study. Patients were scheduled to receive three to four cycles of the neoadjuvant PC (paclitaxel 135 mg m(-2) and cisplatin 75 mg m(-2) on day 1) every 21 days. Patients were then subjected to surgery and subsequently received six cycles of FAC (5-fluorouracil 500 mg m(-2), doxorubicin 50 mg m(-2), and cyclophosphamide 500 mg m(-2)) or four cycles of AC (doxorubicin 60 mg m(-2) and cyclophosphamide 600 mg m(-2)); all drugs were administered intravenously on day 1 with cycles repeated every 21 days. Patients then received radiation therapy, and those with hormone receptor-positive tumours were given adjuvant tamoxifen intended for 5 years. The median age was 41 years. Clinically, 12, 52, and 37% of patients had T2 >4 cm, T3, and T4, respectively. The mean tumour size was 7 cm (95% CI, 7.3-8.5). The clinical nodal status was N0, N1, and N2-N3 in 32, 52, and 17% of patients, respectively. Disease stage at diagnosis was IIA (2%), IIB (32%), IIIA (28%), and IIIB (39%). Clinical assessment of the primary tumour and the axillary nodal status after primary chemotherapy showed that 35 patients (28%) achieved complete response (cCR), while 80 (63%) demonstrated partial response to PC. Of patients with evaluable pathologic data of the primary tumour (123 patients), complete pathologic response (pCR) was achieved in 29 patients (24%), and an additional nine (7%) only had a microinvasive disease. Moreover, 20 of the 122 patients (16%) had no residual disease in the primary tumour or in the axillary nodes. Failure to attain cCR predicted failure to achieve pCR. At a median follow-up of 37.5 months (95% CI, 31.5-43.3), 71% were alive with no recurrence, 16% were alive with evidence of disease, and 13% were dead. Of the 122 patients who had surgery, 36 (29%) developed recurrence including one of the patients who attained pCR. The median overall or disease-free survival has not been reached with a projected 5-year overall survival (OS) and disease-free survival (DFS) of 85% (+/-4%) and 63% (+/-5%), respectively. On multivariate analysis, clinical response of the primary tumour, pathological response of the primary tumour, and the pathological nodal status were identified as independent prognostic variables for DFS. No variable, however, was identified to prognosticate OS. PC was acceptably safe. Neoadjuvant PC as used in this phase II study in a multidisciplinary strategy was highly effective. Clinical and pathologic responses remain the most important variables that predict outcome.
Figures
References
-
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Tereniziani M, Zambetti M (1998) Primary chemotherapy in operable breast cancer. Eight-year experience at Milan Cancer Institute. J Clin Oncol 16: 93–100 - PubMed
-
- Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38: 29–41
-
- Bruckman J, Harris J, Levene M, Chaffey JT, Hellman S (1979) Results of treating stage III carcinoma of the breast by primary radiation therapy. Cancer 43: 985–993 - PubMed
-
- Comella G, Comella P, Scanni A, Apicella G, D’Aiuto G, Thomas R, Capasso I, DiBonito I, Piccolo S, Carteni G, Gravina A, Frasci G (1998) Cisplatin-epirubicin-paclitaxel weekly administration in advanced breast cancer. A phase I study. Proc Am Soc Clin Oncol 17: 510 (abstract) - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical