Concentration-time profile for perhexiline and hydroxyperhexiline in patients at steady state
- PMID: 14998422
- PMCID: PMC1884455
- DOI: 10.1046/j.1365-2125.2003.02003.x
Concentration-time profile for perhexiline and hydroxyperhexiline in patients at steady state
Abstract
Aim: To define inter- and intraday variability in plasma perhexiline concentrations, time-to-maximum plasma perhexiline concentration and variability in the ratio of hydroxyperhexiline to parent perhexiline concentrations over the course of the day in patients at steady state.
Methods: Eight blood samples were taken over a 24-h period from 12 adult patients already taking perhexiline for the treatment of angina pectoris. These patients were assumed to be at steady state, having taken the same dose of perhexiline for more than 4 weeks and having no changes made to other drug therapy that might have affected plasma perhexiline concentrations (especially drugs that interfere with CYP2D6). Perhexiline was assayed by HPLC/FL. The percentage increase over baseline concentration was determined for each patient for both perhexiline and hydroxyperhexiline.
Results: Trough plasma perhexiline concentrations from two patients were below the limit of quantification of the assay (0.05 mg l-1) and thus were excluded from the analysis. The greatest mean percentage increase in plasma perhexiline concentration over the day was 21% (95%CI 9%, 33%, range -19% to 45%) which occurred 6 h postdose. The greatest mean percentage increase in plasma hydroxyperhexiline concentration was 10.8% (95%CI -5.3%, 26.9%, range -13% to 60%) which occurred 4 h postdose. However individual patients demonstrated > 60% intraday variability in perhexiline concentrations which was not related to the concomitant use of drugs that affect CYP2D6 activity. Changes in random plasma perhexiline concentration which are attributed to changes in concomitant drug therapy should be supported by additional kinetic data. Inter-day variability in plasma perhexiline concentration as determined by the ratio of C24 : C0 was small (mean 0.90, 95%CI 0.77, 1.03) which supports C0 as the best sampling time for perhexiline concentration monitoring. The variability in C24 : C0 for hydroxyperhexiline concentrations was smaller (mean 0.96, 95%CI 0.81, 1.11). Variability in the ratio of plasma concentrations of hydroxyperhexiline to perhexiline over the day was also small. The ratio of plasma hydroxyperhexiline to perhexiline concentration over the day fell within a narrow range for all subjects with 95% confidence intervals being < 15% for eight patients and < 25% for the remaining patient. This suggests that formation of the metabolite occurs rapidly and may be presystemic. It also supports the calculation of the hydroxyperhexiline : perhexiline ratio (in patients at steady state) on blood samples taken at any time during the dosing interval.
Conclusions: The within-day variability in plasma perhexiline concentrations was small. While C0 is probably the best time for therapeutic drug monitoring purposes, it is not unreasonable to use samples drawn at any time during the dosing interval. The therapeutic range used in this hospital (0.15-0.6 mg l-1) was devised from earlier work using 4 h postdose blood sampling which is close to the 'peak' concentration and a mean of 16% higher than C0 in this study. This increase is probably clinically insignificant and a different C0 range is therefore not warranted.
Figures
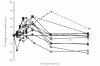
 ), Pt6 (•), Pt7 (♦), Pt8 (
), Pt6 (•), Pt7 (♦), Pt8 ( ), Pt9 (□), Pt10 (○), Pt11 (▵) and Pt12 (♦)
), Pt9 (□), Pt10 (○), Pt11 (▵) and Pt12 (♦)
 ), Pt9 (□), Pt10 (○), Pt11 (▵) and Pt12 (♦)
), Pt9 (□), Pt10 (○), Pt11 (▵) and Pt12 (♦)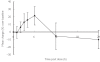
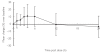
Similar articles
-
Determination of perhexiline and its metabolite hydroxyperhexiline in human plasma by liquid chromatography/tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2009 Oct 1;877(27):3025-30. doi: 10.1016/j.jchromb.2009.07.021. Epub 2009 Jul 21. J Chromatogr B Analyt Technol Biomed Life Sci. 2009. PMID: 19646935
-
Pharmacokinetics of the antianginal agent perhexiline: relationship between metabolic ratio and steady-state dose.Br J Clin Pharmacol. 2002 Aug;54(2):107-14. doi: 10.1046/j.1365-2125.2002.01618.x. Br J Clin Pharmacol. 2002. PMID: 12207628 Free PMC article.
-
Determination of perhexiline and hydroxyperhexiline in plasma by liquid chromatography-mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Jun 5;805(1):87-91. doi: 10.1016/j.jchromb.2004.02.019. J Chromatogr B Analyt Technol Biomed Life Sci. 2004. PMID: 15113543
-
Perhexiline.Cardiovasc Drug Rev. 2007 Spring;25(1):76-97. doi: 10.1111/j.1527-3466.2007.00006.x. Cardiovasc Drug Rev. 2007. PMID: 17445089 Review.
-
Mibefradil, a pharmacologically distinct calcium antagonist.Pharmacotherapy. 1998 May-Jun;18(3):463-85. Pharmacotherapy. 1998. PMID: 9620098 Review.
Cited by
-
A mechanism of perhexiline's cytotoxicity in hepatic cells involves endoplasmic reticulum stress and p38 signaling pathway.Chem Biol Interact. 2021 Jan 25;334:109353. doi: 10.1016/j.cbi.2020.109353. Epub 2020 Dec 9. Chem Biol Interact. 2021. PMID: 33309543 Free PMC article.
References
-
- Armstrong ML, Brand D, Emmett AJ, Hodge JLR, Kelleway GSM, Mesitz P, Refman M, Wallace DC. A multicentre trial of perhexiline maleate, beta-blocker and placebo in angina pectoris. Med J Aust. 1974;2:389–393. - PubMed
-
- Opie LH. Calcium antagonists. Lancet. 1980;i:806–809. - PubMed
-
- Horowitz JD, Button IK, Wing L. Is perhexiline essential for the optimal management of angina pectoris? Aust NZ J Med. 1995;25:111–113. - PubMed
-
- Cooper JDH, Turnell DC, Pilcher J, Lockhart D. Therapeutic monitoring of the antianginal drug perhexiline maleate. Ann Clin Biochem. 1985;22:614–617. - PubMed
-
- Horowitz JD, Pia STB, Macdonald PS, Goble AJ, Louis WJ. Perhexiline maleate treatment for severe angina pectoris – correlations with pharmacokinetics. Int J Cardiol. 1986;13:219–229. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

