Pharmacokinetic evaluation of a new oral sustained release dosage form of tramadol
- PMID: 14998423
- PMCID: PMC1884445
- DOI: 10.1046/j.1365-2125.2003.02013.x
Pharmacokinetic evaluation of a new oral sustained release dosage form of tramadol
Abstract
Aims: To compare the pharmacokinetic profile of a new modified release formulation of tramadol (Tramadol LP 200 mg, SMB Technology, Marche-en-Famenne, Belgium) with that of an immediate release capsule (Topalgic) 50 mg, Grünenthal, Aachen, Germany) after single and multiple dosing and to assess the potential effect of food on its relative bioavailability.
Methods: The first study had an open, single-dose, three-treatment, three-period, six-sequence, randomised, crossover design with at least a five-day wash-out. The second study had an open, steady-state, two-treatment, two-period, two-sequence, randomised crossover design with at least a seven-day wash-out. Both studies contained 30 healthy subjects. Both enantiomers of tramadol and O-demethyl-tramadol (the only active metabolite of tramadol) were assayed in the plasma using an LC-MS/MS method. AUC infinity, AUCt, Cmax, Tmax, and T1/2 were estimated. Statistical analysis was performed using univariate anova, the Wilcoxon nonparametric method or Friedman's nonparametric anova where appropriate.
Results: Tramadol had a significantly lower Cmax and longer Tmax than the conventional formulation. Thus, the mean (+/- sd) Cmax of tramadol were 646 +/- 192 and 300 +/- 94 ng ml-1 for Topalgic 4 x 50mg and Tramadol LP 200 mg, respectively (95% confidence interval on the difference expressed as a percentage 42-51). AUC of tramadol from both formulations was comparable (similar AUC infinity and AUCt). Thus, the mean AUC infinity of (+/-)tramadol obtained after multiple dosing were 4611 +/- 1944 and 5105 +/- 2101 ngh ml-1 after Topalgic 4 x 50mg and Tramadol LP 200 mg, respectively (95%CI 102-123%). We also demonstrate that the pharmacokinetics of the drug are not influenced by the intake of food. Thus, the mean AUC infinity of (+/-) tramadol were 5444 +/- 1637 and 5169 +/- 1580 ngh ml-1 after Tramadol LP 200 mg given in the fasting and fed states, respectively (95%CI = 88-103%).
Conclusions: The new sustained release form of tramadol exhibits adequate properties for once a day administration. Furthermore, its pharmacokinetic profile is not affected by the intake of food.
Figures
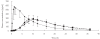
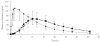
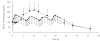


Similar articles
-
Comparative bioavailability between two Tramadol once-daily oral formulations.Methods Find Exp Clin Pharmacol. 2006 Jul-Aug;28(6):373-8. doi: 10.1358/mf.2006.28.6.1007674. Methods Find Exp Clin Pharmacol. 2006. PMID: 16894407 Clinical Trial.
-
Pharmacokinetic properties of tramadol sustained release capsules. 2nd communication: investigation of relative bioavailability and food interaction.Arzneimittelforschung. 1999 Jul;49(7):588-93. doi: 10.1055/s-0031-1300467. Arzneimittelforschung. 1999. PMID: 10442206 Clinical Trial.
-
A Randomized, Crossover Study on the Effect of Food on the Pharmacokinetic Characteristics of Morphine ARER (MorphaBond™ ER), an Abuse-Deterrent Formulation of Extended-Release Morphine.Adv Ther. 2019 Sep;36(9):2394-2401. doi: 10.1007/s12325-019-01022-4. Epub 2019 Jul 5. Adv Ther. 2019. PMID: 31278694 Free PMC article. Clinical Trial.
-
Sustained relief of chronic pain. Pharmacokinetics of sustained release morphine.Clin Pharmacokinet. 1998 Sep;35(3):173-90. doi: 10.2165/00003088-199835030-00002. Clin Pharmacokinet. 1998. PMID: 9784932 Review.
-
Tramadol sustained-release capsules.Drugs. 2006;66(2):223-30. doi: 10.2165/00003495-200666020-00006. Drugs. 2006. PMID: 16451094 Review.
Cited by
-
Population pharmacokinetic modeling of tramadol and its O-desmethyl metabolite in plasma and breast milk.Eur J Clin Pharmacol. 2011 Sep;67(9):899-908. doi: 10.1007/s00228-011-1023-6. Epub 2011 Mar 11. Eur J Clin Pharmacol. 2011. PMID: 21394525
-
Review of extended-release formulations of Tramadol for the management of chronic non-cancer pain: focus on marketed formulations.J Pain Res. 2014 Mar 24;7:149-61. doi: 10.2147/JPR.S49502. eCollection 2014. J Pain Res. 2014. PMID: 24711710 Free PMC article. Review.
-
O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-μ-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells.Int J Mol Sci. 2025 Apr 27;26(9):4139. doi: 10.3390/ijms26094139. Int J Mol Sci. 2025. PMID: 40362379 Free PMC article.
-
Pharmacokinetic and urine profile of tramadol and its major metabolites following oral immediate release capsules administration in dogs.Vet Res Commun. 2009 Dec;33(8):875-85. doi: 10.1007/s11259-009-9236-1. Vet Res Commun. 2009. PMID: 19533402
-
Tapentadol Versus Tramadol: A Narrative and Comparative Review of Their Pharmacological, Efficacy and Safety Profiles in Adult Patients.Drugs. 2021 Jul;81(11):1257-1272. doi: 10.1007/s40265-021-01515-z. Epub 2021 Jul 1. Drugs. 2021. PMID: 34196947 Free PMC article.
References
-
- Lee CR, McTavish D, Sorkin EM. Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs. 1993;46:313–340. - PubMed
-
- Collart L, Luthy C, Favario-Constantin C, Dayer P. [Duality of the analgesic effect of tramadol in humans] Schweiz Med. 1993;123:2241–2243. - PubMed
-
- Dayer P, Collart L, Desmeules J. The pharmacology of tramadol. Drugs. 1994;47(Suppl 1):3–7. - PubMed
-
- Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41:7–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

