Transverse propagation of action potentials between parallel chains of cardiac muscle and smooth muscle cells in PSpice simulations
- PMID: 14998434
- PMCID: PMC400751
- DOI: 10.1186/1475-925X-3-5
Transverse propagation of action potentials between parallel chains of cardiac muscle and smooth muscle cells in PSpice simulations
Abstract
Background: We previously examined transverse propagation of action potentials between 2 and 3 parallel chain of cardiac muscle cells (CMC) simulated using the PSpice program. The present study was done to examine transverse propagation between 5 parallel chains in an expanded model of CMC and smooth muscle cells (SMC).
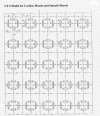
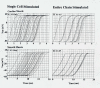
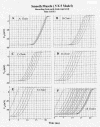
Methods: Excitation was transmitted from cell to cell along a strand of 5 cells not connected by low-resistance tunnels (gap-junction connexons). The entire surface membrane of each cell fired nearly simultaneously, and nearly all the propagation time was spent at the cell junctions, the junctional delay time being about 0.3-0.5 ms (CMC) or 0.8-1.6 ms (SMC). A negative cleft potential (Vjc) develops in the narrow junctional clefts, whose magnitude depends on the radial cleft resistance (Rjc), which depolarizes the postjunctional membrane (post-JM) to threshold. Propagation velocity (theta) increased with amplitude of Vjc. Therefore, one mechanism for the transfer of excitation from one cell to the next is by the electric field (EF) that is generated in the junctional cleft when the pre-JM fires. In the present study, 5 parallel stands of 5 cells each (5 x 5 model) were used.
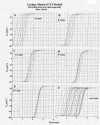
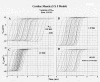
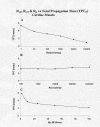
Results: With electrical stimulation of the first cell of the first strand (cell A1), propagation rapidly spread down that chain and then jumped to the second strand (B chain), followed by jumping to the third, fourth, and fifth strands (C, D, E chains). The rapidity by which the parallel chains became activated depended on the longitudinal resistance of the narrow extracellular cleft between the parallel strands (Rol2); the higher the Rol2 resistance, the faster the theta. The transverse resistance of the cleft (Ror2) had almost no effect. Increasing Rjc decreases the total propagation time (TPT) over the 25-cell network. When the first cell of the third strand (cell C1) was stimulated, propagation spread down the C chain and jumped to the other two strands (B and D) nearly simultaneously.
Conclusions: Transverse propagation of excitation occurred at multiple points along the chain as longitudinal propagation was occurring, causing the APs in the contiguous chains to become bunched up. Transverse propagation was more erratic and labile in SMC compared to CMC. Transverse transmission of excitation did not require low-resistance connections between the chains, but instead depended on the value of Rol2. The tighter the packing of the chains facilitated transverse propagation.
Figures




Similar articles
-
Propagation of action potentials between parallel chains of cardiac muscle cells in PSpice simulation.Can J Physiol Pharmacol. 2003 Jan;81(1):48-58. doi: 10.1139/y03-019. Can J Physiol Pharmacol. 2003. PMID: 12665257
-
Effect of transverse gap-junction channels on transverse propagation in an enlarged PSpice model of cardiac muscle.Theor Biol Med Model. 2006 Mar 16;3:14. doi: 10.1186/1742-4682-3-14. Theor Biol Med Model. 2006. PMID: 16542447 Free PMC article.
-
Combined electric field and gap junctions on propagation of action potentials in cardiac muscle and smooth muscle in PSpice simulation.J Electrocardiol. 2003 Oct;36(4):279-93. doi: 10.1016/j.jelectrocard.2003.08.001. J Electrocardiol. 2003. PMID: 14661164
-
Role of gap junctions in the propagation of the cardiac action potential.Cardiovasc Res. 2004 May 1;62(2):309-22. doi: 10.1016/j.cardiores.2003.11.035. Cardiovasc Res. 2004. PMID: 15094351 Review.
-
Basic mechanisms of cardiac impulse propagation and associated arrhythmias.Physiol Rev. 2004 Apr;84(2):431-88. doi: 10.1152/physrev.00025.2003. Physiol Rev. 2004. PMID: 15044680 Review.
Cited by
-
Cable properties and propagation velocity in a long single chain of simulated myocardial cells.Theor Biol Med Model. 2007 Sep 14;4:36. doi: 10.1186/1742-4682-4-36. Theor Biol Med Model. 2007. PMID: 17868460 Free PMC article.
-
Boundary effects influence velocity of transverse propagation of simulated cardiac action potentials.Theor Biol Med Model. 2005 Sep 6;2:36. doi: 10.1186/1742-4682-2-36. Theor Biol Med Model. 2005. PMID: 16144554 Free PMC article.
-
Gap-junction channels inhibit transverse propagation in cardiac muscle.Biomed Eng Online. 2005 Jan 28;4:7. doi: 10.1186/1475-925X-4-7. Biomed Eng Online. 2005. PMID: 15679888 Free PMC article.
-
Transverse propagation in an expanded PSpice model for cardiac muscle with gap-junction ion channels.Biomed Eng Online. 2006 Jul 28;5:46. doi: 10.1186/1475-925X-5-46. Biomed Eng Online. 2006. PMID: 16875501 Free PMC article.
References
-
- Sperelakis N, Mann JE. Evaluation of electric field changes in the cleft between excitable cells. J Theor Biol. 1977;64:71–96. - PubMed
-
- Sperelakis N, McConnell K. An electric field mechanism for transmission of excitation from cell to cell in cardiac muscle and smooth muscles. In: Mohan RM, editor. Research Advances in Biomedical Engineering. Vol. 2. Global Research Network; 2001. pp. 39–66.
-
- Sperelakis N. Cell Physiology Source Book. 1. Academic Press Publishers; 1995. Cable Properties and propagation of action potentials, ch. 18; pp. 245–254.
-
- Cohen SA. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation. 1996;94:3083–3086. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

