Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents
- PMID: 14998492
- PMCID: PMC4217165
- DOI: 10.1016/s1535-6108(04)00020-0
Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents
Abstract
The MUC1 transforming protein is overexpressed by most human carcinomas. The present studies demonstrate that the MUC1 C-terminal subunit (MUC1 C-ter) localizes to mitochondria in HCT116/MUC1 colon carcinoma cells and that heregulin stimulates mitochondrial targeting of MUC1 C-ter. We also show that MUC1 attenuates cisplatin-induced (1) release of mitochondrial apoptogenic factors, (2) activation of caspase-3, and (3) induction of apoptosis. Moreover, knockdown of MUC1 expression in A549 lung and ZR-75-1 breast carcinoma cells by MUC1siRNA was associated with increased sensitivity to genotoxic drugs in vitro and in vivo. These findings indicate that MUC1 attenuates the apoptotic response to DNA damage and that this oncoprotein confers resistance to genotoxic anticancer agents.
Figures



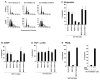
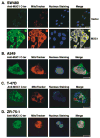

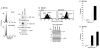

Comment in
-
Protein hijacking: key proteins held captive against their will.Cancer Cell. 2004 Feb;5(2):107-8. doi: 10.1016/s1535-6108(04)00029-7. Cancer Cell. 2004. PMID: 14998483 Review.
References
-
- Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–1475. - PubMed
-
- Boldin M, Goncharov T, Goltsev Y, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell-death. Cell. 1996;85:803–815. - PubMed
-
- Bunz F. Cell death and cancer therapy. Curr Opin Pharmacol. 2001;1:337–341. - PubMed
-
- Datta R, Manome Y, Taneja N, Boise LH, Weichselbaum RR, Thompson CB, Slapak CA, Kufe DW. Overexpression of Bcl-xL by cytotoxic drug exposure confers resistance to ionizing radiation-induced internucleosomal DNA fragmentation. Cell Growth Differ. 1995;6:363–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

