Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei
- PMID: 14999061
- PMCID: PMC6730417
- DOI: 10.1523/JNEUROSCI.3988-03.2004
Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei
Abstract
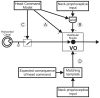
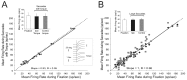
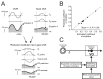
The ability to distinguish sensory inputs that are a consequence of our own actions from those that result from changes in the external world is essential for perceptual stability and accurate motor control. To accomplish this, it has been proposed that an internal prediction of the consequences of our actions is compared with the actual sensory input to cancel the resultant self-generated activation. Here, we provide evidence for this hypothesis at an early stage of processing in the vestibular system. Previous studies have shown that neurons in the vestibular nucleus, which receive direct inputs from vestibular afferent fibers, are responsive to passively applied head movements. However, these same neurons do not reliably encode head velocity resulting from self-generated movements of the head on the body. In this study, we examined the mechanism that underlies the selective elimination of vestibular sensitivity to active head-on-body rotations. Individual neurons were recorded in monkeys making active head movements. The correspondence between intended and actual head movement was experimentally controlled. We found that a cancellation signal was gated into the vestibular nuclei only in conditions in which the activation of neck proprioceptors matched that expected on the basis of the neck motor command. This finding suggests that vestibular signals that arise from self-generated head movements are inhibited by a mechanism that compares the internal prediction of the sensory consequences by the brain to the actual resultant sensory feedback. Because self-generated vestibular inputs are selectively cancelled early in processing, we propose that this gating is important for the computation of spatial orientation and control of posture by higher-order structures.
Figures





References
-
- Akbarian S, Grüsser O-J, Guldin WO (1993) Corticofugal projections to the vestibular nuclei in squirrel monkeys: further evidence of multiple cortical vestibular fields. J Comp Neurol 332: 89-104. - PubMed
-
- Akbarian S, Grüsser O-J, Guldin WO (1994) Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol 339: 421-437. - PubMed
-
- Alstermark B, Pinter MJ, Sasaki S (1992a) Descending pathways mediating disynaptic excitation of dorsal neck motoneurons in the cat: facilitatory interactions. Neurosci Res 15: 32-41. - PubMed
-
- Alstermark B, Pinter MJ, Sasaki S (1992b) Descending pathways mediating disynaptic excitation of dorsal neck motoneurons in the cat: brain stem relay. Neurosci Res 15: 42-57. - PubMed
-
- Anastasopoulos D, Mergner T (1982) Canal-neck interaction in vestibular nuclear neurons of the cats. Exp Brain Res 46: 269-280. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
