Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice
- PMID: 14999075
- PMCID: PMC6730438
- DOI: 10.1523/JNEUROSCI.5285-03.2004
Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice
Abstract
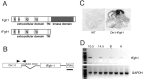
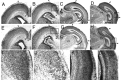
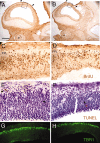
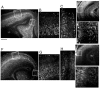
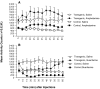
Fibroblast growth factor receptor (FGFR) gene products (Fgfr1, Fgfr2, Fgfr3) are widely expressed by embryonic neural progenitor cells throughout the CNS, yet their functional role in cerebral cortical development is still unclear. To understand whether the FGF pathways play a role in cortical development, we attenuated FGFR signaling by expressing a tyrosine kinase domain-deficient Fgfr1 (tFgfr1) gene construct during embryonic brain development. Mice carrying the tFgfr1 transgene under the control of the Otx1 gene promoter have decreased thickness of the cerebral cortex in frontal and temporal areas because of decreased number of pyramidal neurons and disorganization of pyramidal cell dendritic architecture. These alterations may be, in part, attributable to decreased genesis of T-Brain-1-positive early glutamatergic neurons and, in part, to a failure to maintain radial glia fibers in medial prefrontal and temporal areas of the cortical plate. No changes were detected in cortical GABAergic interneurons, including Cajal-Retzius cells or in the basal ganglia. Behaviorally, tFgfr1 transgenic mice displayed spontaneous and persistent locomotor hyperactivity that apparently was not attributable to alterations in subcortical monoaminergic systems, because transgenic animals responded to both amphetamine and guanfacine, an alpha2A adrenergic receptor agonist. We conclude that FGF tyrosine kinase signaling may be required for the genesis and growth of pyramidal neurons in frontal and temporal cortical areas, and that alterations in cortical development attributable to disrupted FGF signaling are critical for the inhibitory regulation of motor behavior.
Figures



References
-
- Amaya E, Musci TJ, Kirschner MW (1991) Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66: 257-270. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL (1997) Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278: 474-476. - PubMed
-
- Anton ES, Marchionni MA, Lee KF, Rakic P (1997) Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124: 3501-3510. - PubMed
-
- Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA, Bluethmann H, Kew JN (2002) Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci 22: 6713-6723. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
