Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis
- PMID: 14999076
- PMCID: PMC6730423
- DOI: 10.1523/JNEUROSCI.5191-03.2004
Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis
Abstract
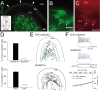
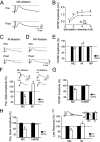
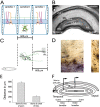
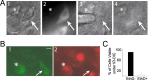
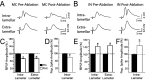
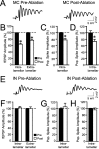
Loss of cells from the hilus of the dentate gyrus is a major histological hallmark of human temporal lobe epilepsy. Hilar mossy cells, in particular, are thought to show dramatic numerical reductions in pathological conditions, and one prominent theory of epileptogenesis is based on the assumption that mossy cell loss directly results in granule cell hyperexcitability. However, whether it is the disappearance of hilar mossy cells from the dentate gyrus circuitry after various insults or the subsequent synaptic-cellular alterations (e.g., reactive axonal sprouting) that lead to dentate hyperexcitability has not been rigorously tested, because of the lack of available techniques to rapidly remove specific classes of nonprincipal cells from neuronal networks. We developed a fast, cell-specific ablation technique that allowed the targeted lesioning of either mossy cells or GABAergic interneurons in horizontal as well as axial (longitudinal) slices of the hippocampus. The results demonstrate that mossy cell deletion consistently decreased the excitability of granule cells to perforant path stimulation both within and outside of the lamella where the mossy cell ablation took place. In contrast, ablation of interneurons caused the expected increase in excitability, and control aspirations of the hilar neuropil or of interneurons in the presence of GABA receptor blockers caused no alteration in granule cell excitability. These data do not support the hypothesis that loss of mossy cells from the dentate hilus after seizures or traumatic brain injury directly results in hyperexcitability.
Figures
References
-
- Amaral DG (1978) A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol 182: 851-914. - PubMed
-
- Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571-591. - PubMed
-
- Andersen P, Soleng AF, Raastad M (2000) The hippocampal lamella hypothesis revisited. Brain Res 886: 165-171. - PubMed
-
- Arabadzisz D, Freund TF (1999) Changes in excitatory and inhibitory circuits of the rat hippocampus 12-14 months after complete forebrain ischemia. Neuroscience 92: 27-45. - PubMed
-
- Bernard C, Esclapez M, Hirsch JC, Ben Ari Y (1998) Interneurones are not so dormant in temporal lobe epilepsy: a critical reappraisal of the dormant basket cell hypothesis. Epilepsy Res 32: 93-103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
