Solid phase synthesis and binding affinity of peptidyl transferase transition state mimics containing 2'-OH at P-site position A76
- PMID: 14999092
- PMCID: PMC390298
- DOI: 10.1093/nar/gkh311
Solid phase synthesis and binding affinity of peptidyl transferase transition state mimics containing 2'-OH at P-site position A76
Abstract
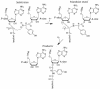
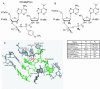
All living cells are dependent on ribosomes to catalyze the peptidyl transfer reaction, by which amino acids are assembled into proteins. The previously studied peptidyl transferase transition state analog CC-dA-phosphate-puromycin (CCdApPmn) has important differences from the transition state, yet current models of the ribosomal active site have been heavily influenced by the properties of this molecule. One significant difference is the substitution of deoxyadenosine for riboadenosine at A76, which mimics the 3' end of a P-site tRNA. We have developed a solid phase synthetic approach to produce inhibitors that more closely match the transition state, including the critical P-site 2'-OH. Inclusion of the 2'-OH or an even bulkier OCH3 group causes significant changes in binding affinity. We also investigated the effects of changing the A-site amino acid side chain from phenylalanine to alanine. These results indicate that the absence of the 2'-OH is likely to play a significant role in the binding and conformation of CCdApPmn in the ribosomal active site by eliminating steric clash between the 2'-OH and the tetrahedral phosphate oxygen. The conformation of the actual transition state must allow for the presence of the 2'-OH, and transition state mimics that include this critical hydroxyl group must bind in a different conformation from that seen in prior analog structures. These new inhibitors will provide valuable insights into the geometry and mechanism of the ribosomal active site.
Figures


References
-
- Chladek S. and Sprinzl,M. (1985) The 3′-end of transfer-RNA and its role in protein biosynthesis. Angew. Chem. Int. Ed., 24, 371–391.
-
- Yarus M. and Welch,M. (2000) Peptidyl transferase: ancient and exiguous. Chem. Biol., 7, R187–R190. - PubMed
-
- Green R. and Noller,H.F. (1997) Ribosomes and translation. Annu. Rev. Biochem., 66, 679–716. - PubMed
-
- Ban N., Nissen,P., Hansen,J., Moore,P.B. and Steitz,T.A. (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science, 289, 905–920. - PubMed

