A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor
- PMID: 14999100
- PMCID: PMC374319
- DOI: 10.1073/pnas.0307713101
A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor
Abstract
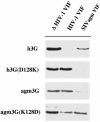
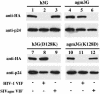
The HIV type 1 (HIV-1) virion infectivity factor (Vif) protein blocks the action of the host defense factor APOBEC3G in human cells, thereby allowing release of infectious virions, but fails to inhibit similar APOBEC3G proteins present in some simian cells. Conversely, the Vif protein encoded by the African green monkey (agm) simian immunodeficiency virus (SIV) can block agm APOBEC3G function but fails to inhibit human APOBEC3G. This difference plays a key role in determining the primate species tropism of HIV-1 and SIV agm. Here, we demonstrate that a single APOBEC3G residue, which is an aspartic acid in human APOBEC3G and a lysine in agm APOBEC3G, controls the ability of the HIV-1 Vif protein to bind and inactivate these host defense factors. These data identify a critical charged residue that plays a key role in mediating the formation of the distinct Vif:APOBEC3G complexes formed in human and simian cells. Moreover, these results suggest that the biological barrier preventing the entry of additional SIV into the human population as zoonotic infections is potentially quite fragile.
Figures



Comment in
-
Controlling lentiviruses: single amino acid changes can determine specificity.Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3725-6. doi: 10.1073/pnas.0400929101. Epub 2004 Mar 9. Proc Natl Acad Sci U S A. 2004. PMID: 15010528 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

