Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect
- PMID: 15001702
- PMCID: PMC374345
- DOI: 10.1073/pnas.0400380101
Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect
Abstract
Acute graft-versus-host disease (GVHD) and leukemic relapse are the two major obstacles to successful outcomes after allogeneic bone marrow transplantation (BMT), an effective therapy for hematological malignancies. Several studies have demonstrated that the dysregulation of proinflammatory cytokines and the loss of gastrointestinal tract integrity contribute to GVHD, whereas the donor cytotoxic responses are critical for graft-versus-leukemia (GVL) preservation. Suberoylanilide hydroxamic acid (SAHA) is currently in clinical trials as an antitumor agent; it inhibits the activity of histone deacetylases and at low doses exhibits antiinflammatory effects by reducing the production of proinflammatory cytokines. Using two well characterized mouse models of BMT, we have studied the effects of SAHA on GVHD severity and GVL activity. Administration of SAHA from day +3 to day +7 after BMT reduced serum levels of the proinflammatory cytokines and decreased intestinal histopathology, clinical severity, and mortality from acute GVHD compared with vehicle-treated animals. However, SAHA had no effect on donor T cell proliferative and cytotoxic responses to host antigens in vivo or in vitro. When mice received lethal doses of tumor cells at the time of BMT, administration of SAHA did not impair GVL activity and resulted in significantly improved leukemia-free survival by using two different tumor and donor/recipient combinations. These findings reveal a critical role for histone deacetylase inhibition in the proinflammatory events contributing to GVHD and suggest that this class of pharmacologic agents may provide a strategy to reduce GVHD while preserving cytotoxic T cell responses to host antigens and maintaining beneficial GVL effects.
Figures

 ); *, P < 0.05. Results from one of two similar experiments are shown. (b) Serum IL1-β levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients (
); *, P < 0.05. Results from one of two similar experiments are shown. (b) Serum IL1-β levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients ( ); **, P < 0.02. Data are from one of two similar experiments. (c) Serum IFN-γ levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients (
); **, P < 0.02. Data are from one of two similar experiments. (c) Serum IFN-γ levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients ( ); **, P < 0.02. Data from one of three similar experiments are shown.
); **, P < 0.02. Data from one of three similar experiments are shown.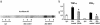
 , SAHA allo recipients) after BMT as above. The expression of TNF-α and IFN-γ mRNA levels were analyzed by RPA, as described in Materials and Methods. The TNF-α and the IFN-γ mRNA transcript levels were reduced in the recipients treated with SAHA. Shown are controls (▪) vs. SAHA allo recipients (
, SAHA allo recipients) after BMT as above. The expression of TNF-α and IFN-γ mRNA levels were analyzed by RPA, as described in Materials and Methods. The TNF-α and the IFN-γ mRNA transcript levels were reduced in the recipients treated with SAHA. Shown are controls (▪) vs. SAHA allo recipients ( ); *, P < 0.05. Results are from one of two similar experiments.
); *, P < 0.05. Results are from one of two similar experiments.
 , n = 4) from allo donors and of syngeneic donors (□, n = 4) are shown. Total GVHD score: mean ± SE of the sum of scores for small bowel and colon from individual animals in each group. *, P < 0.05, control (▪) vs. the SAHA (
, n = 4) from allo donors and of syngeneic donors (□, n = 4) are shown. Total GVHD score: mean ± SE of the sum of scores for small bowel and colon from individual animals in each group. *, P < 0.05, control (▪) vs. the SAHA ( ) allo group.
) allo group.

 ) vs. allo plus SAHA (▪), P = NS]. Recipients of syngeneic BMT (□) demonstrated lower numbers of donor T cells at both time points. Data from one of three similar experiments are shown. (c) Harvested splenocytes normalized for donor T cells (CD45.1+ and CD3+) were restimulated in quadruplicate with irradiated naive host (syngeneic B6 Ly5.2 and allo F1) splenocytes in mixed lymphocyte reaction cultures for 48 h. Proliferation was determined by incubation of cells with [3H]thymidine (1 μCi per well) for an additional 24 h. T cells from SAHA-treated animals showed equivalent proliferation (
) vs. allo plus SAHA (▪), P = NS]. Recipients of syngeneic BMT (□) demonstrated lower numbers of donor T cells at both time points. Data from one of three similar experiments are shown. (c) Harvested splenocytes normalized for donor T cells (CD45.1+ and CD3+) were restimulated in quadruplicate with irradiated naive host (syngeneic B6 Ly5.2 and allo F1) splenocytes in mixed lymphocyte reaction cultures for 48 h. Proliferation was determined by incubation of cells with [3H]thymidine (1 μCi per well) for an additional 24 h. T cells from SAHA-treated animals showed equivalent proliferation ( vs.▪, P = NS). Results from one of three similar experiments are shown. (d) SAHA treatment preserves cytotoxic T lymphocyte function after BMT. Splenocytes harvested from allo animals on day +14 after BMT were pooled (n = 3 per group), normalized for donor CD8+ cells, and used in a 51Cr-release assay. Cytotoxic T lymphocyte activity against allo targets (P815) in control (
vs.▪, P = NS). Results from one of three similar experiments are shown. (d) SAHA treatment preserves cytotoxic T lymphocyte function after BMT. Splenocytes harvested from allo animals on day +14 after BMT were pooled (n = 3 per group), normalized for donor CD8+ cells, and used in a 51Cr-release assay. Cytotoxic T lymphocyte activity against allo targets (P815) in control ( ) and SAHA (▪) groups was similar, whereas no significant lysis of syngeneic targets (EL-4) by both groups (▴ and ▾) occurred. Data from one of two similar experiments are shown.
) and SAHA (▪) groups was similar, whereas no significant lysis of syngeneic targets (EL-4) by both groups (▴ and ▾) occurred. Data from one of two similar experiments are shown.
Similar articles
-
LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation.J Clin Invest. 2001 Jun;107(12):1581-9. doi: 10.1172/JCI12156. J Clin Invest. 2001. PMID: 11413166 Free PMC article.
-
Valproic Acid Ameliorates Graft-versus-Host Disease by Downregulating Th1 and Th17 Cells.J Immunol. 2015 Aug 15;195(4):1849-57. doi: 10.4049/jimmunol.1500578. Epub 2015 Jul 15. J Immunol. 2015. PMID: 26179902
-
Reduction of graft-versus-host disease by histone deacetylase inhibitor suberonylanilide hydroxamic acid is associated with modulation of inflammatory cytokine milieu and involves inhibition of STAT1.Exp Hematol. 2006 Jun;34(6):776-87. doi: 10.1016/j.exphem.2006.02.014. Exp Hematol. 2006. PMID: 16728283
-
Alloreactivity and the predictive value of anti-recipient specific interleukin 2 producing helper T lymphocyte precursor frequencies for alloreactivity after bone marrow transplantation.Dan Med Bull. 2002 May;49(2):89-108. Dan Med Bull. 2002. PMID: 12064093 Review.
-
The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease.J Endotoxin Res. 2002;8(6):441-8. doi: 10.1179/096805102125001046. J Endotoxin Res. 2002. PMID: 12697087 Review.
Cited by
-
BET-bromodomain and EZH2 inhibitor-treated chronic GVHD mice have blunted germinal centers with distinct transcriptomes.Blood. 2022 May 12;139(19):2983-2997. doi: 10.1182/blood.2021014557. Blood. 2022. PMID: 35226736 Free PMC article.
-
The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo.Mol Med. 2005 Jan-Dec;11(1-12):1-15. doi: 10.2119/2006-00005.Dinarello. Mol Med. 2005. PMID: 16557334 Free PMC article.
-
Meta-Analysis of Genome-Wide Association and Gene Expression Studies Implicates Donor T Cell Function and Cytokine Pathways in Acute GvHD.Front Immunol. 2020 Feb 3;11:19. doi: 10.3389/fimmu.2020.00019. eCollection 2020. Front Immunol. 2020. PMID: 32117222 Free PMC article.
-
Differential susceptibility of C57BL/6NCr and B6.Cg-Ptprca mice to commensal bacteria after whole body irradiation in translational bone marrow transplant studies.J Transl Med. 2008 Feb 28;6:10. doi: 10.1186/1479-5876-6-10. J Transl Med. 2008. PMID: 18307812 Free PMC article.
-
Epigenetic targets for reversing immune defects caused by alcohol exposure.Alcohol Res. 2013;35(1):97-113. Alcohol Res. 2013. PMID: 24313169 Free PMC article. Review.
References
-
- Appelbaum, F. R. (2001) Nature 411, 385-389. - PubMed
-
- Antin, J. H. & Ferrara, J. L. M. (1992) Blood 80, 2964-2968. - PubMed
-
- Teshima, T., Ordemann, R., Reddy, P., Gagin, S., Liu, C., Cooke, K. R. & Ferrara, J. L. (2002) Nat. Med. 8, 575-581. - PubMed
-
- Reddy, P. & Ferrara, J. L. (2003) Blood Rev. 17, 187-194. - PubMed
-
- Riddell, S. R., Murata, M., Bryant, S. & Warren, E. H. (2002) Int. J. Hematol. 76, Suppl. 2, 155-161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

