Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export
- PMID: 15001708
- PMCID: PMC374349
- DOI: 10.1073/pnas.0307223101
Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export
Abstract
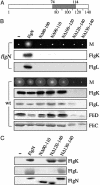
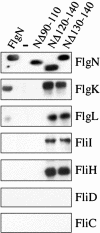
Bacterial type III protein export underlies flagellum assembly and delivery of virulence factors into eukaryotic cells. The sequence of protein interactions underlying the export pathway are poorly characterized; in particular, it is not known how chaperoned substrates in the cytosol are engaged by the membrane-localized export apparatus. We have identified a stalled intermediate export complex in the flagellar type III export pathway of Salmonella typhimurium by generating dominant-negative chaperone variants that are export-defective and arrest flagellar assembly in the wild-type bacterium. These chaperone variants bound their specific export substrates strongly and severely reduced their export. They also attenuated export of other flagellar proteins, indicating that inhibition occurs at a common step in the pathway. Unlike the cytosolic wild-type chaperone, the variants localized to the inner membrane, but not in the absence of the flagellar type III export apparatus. Membrane localization persisted in fliOPQR, flhB, flhA, fliJ, and fliH null mutants lacking specific flagellar export components but depended on the presence of the membrane-associated ATPase FliI. After expression of the variant chaperones in Salmonella, a stalled intermediate export complex, which contained chaperone, substrate, and the FliI ATPase with its regulator FliH, was isolated. Neither chaperone nor substrate alone was able to interact with liposome-associated FliI, but the chaperone-substrate-FliI(FliH) complex was assembled when chaperone was prebound to its substrate. Our data establish a key event in the type III protein export mechanism, docking of the cytosolic chaperone-substrate complex at the ATPase of the membrane-export apparatus.
Figures



Similar articles
-
Interactions between C ring proteins and export apparatus components: a possible mechanism for facilitating type III protein export.Mol Microbiol. 2006 May;60(4):984-98. doi: 10.1111/j.1365-2958.2006.05149.x. Mol Microbiol. 2006. PMID: 16677309
-
Functional defect and restoration of temperature-sensitive mutants of FlhA, a subunit of the flagellar protein export apparatus.J Mol Biol. 2012 Feb 3;415(5):855-65. doi: 10.1016/j.jmb.2011.12.007. Epub 2011 Dec 9. J Mol Biol. 2012. PMID: 22178139
-
Interaction between FliI ATPase and a flagellar chaperone FliT during bacterial flagellar protein export.Mol Microbiol. 2012 Jan;83(1):168-78. doi: 10.1111/j.1365-2958.2011.07924.x. Epub 2011 Dec 7. Mol Microbiol. 2012. PMID: 22111876
-
Protein export through the bacterial flagellar type III export pathway.Biochim Biophys Acta. 2014 Aug;1843(8):1642-8. doi: 10.1016/j.bbamcr.2013.09.005. Epub 2013 Sep 21. Biochim Biophys Acta. 2014. PMID: 24064315 Review.
-
[Structure and function of the bacterial flagellar type III protein export system in Salmonella ].Nihon Saikingaku Zasshi. 2015;70(3):351-64. doi: 10.3412/jsb.70.351. Nihon Saikingaku Zasshi. 2015. PMID: 26310179 Review. Japanese.
Cited by
-
A positive charge region of Salmonella FliI is required for ATPase formation and efficient flagellar protein export.Commun Biol. 2021 Apr 12;4(1):464. doi: 10.1038/s42003-021-01980-y. Commun Biol. 2021. PMID: 33846530 Free PMC article.
-
Crystallization and preliminary X-ray analysis of Salmonella FliI, the ATPase component of the type III flagellar protein-export apparatus.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006 Oct 1;62(Pt 10):973-5. doi: 10.1107/S1744309106033100. Epub 2006 Sep 19. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006. PMID: 17012787 Free PMC article.
-
Building a flagellum outside the bacterial cell.Trends Microbiol. 2014 Oct;22(10):566-72. doi: 10.1016/j.tim.2014.05.009. Epub 2014 Jun 24. Trends Microbiol. 2014. PMID: 24973293 Free PMC article. Review.
-
Components of the Legionella pneumophila flagellar regulon contribute to multiple virulence traits, including lysosome avoidance and macrophage death.Infect Immun. 2005 Sep;73(9):5720-34. doi: 10.1128/IAI.73.9.5720-5734.2005. Infect Immun. 2005. PMID: 16113289 Free PMC article.
-
Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits.Proc Natl Acad Sci U S A. 2007 Jan 9;104(2):485-90. doi: 10.1073/pnas.0608090104. Epub 2007 Jan 3. Proc Natl Acad Sci U S A. 2007. PMID: 17202259 Free PMC article.
References
-
- Macnab, R. M. (1996) in Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology, ed. Neidhardt, F. C. (Am. Soc. Microbiol., Washington, DC), pp. 123-145.
-
- Macnab, R. M. (2003) Annu. Rev. Microbiol. 57, 77-100. - PubMed
-
- Yonekura, K., Maki, S., Morgan, D. G., DeRosier, D. J., Vonderviszt, F., Imada, K. & Namba, K. (2000) Science 290, 2148-2152. - PubMed
-
- Yonekura, K., Maki-Yonekura, S. & Namba, K. (2003) Nature 424, 643-650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

