Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis
- PMID: 15003265
- PMCID: PMC5875116
- DOI: 10.1016/j.pep.2003.12.016
Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis
Abstract
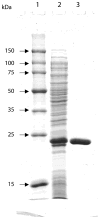
Azo dyes represent a major class of synthetic colorants that are ubiquitous in foods and consumer products. Enterococcus faecalis is a predominant member of the human gastrointestinal microflora. Strain ATCC 19433 grew in the presence of azo dyes and metabolized them to colorless products. A gene encoding a putative FMN-dependent aerobic azoreductase that shares 34% identity with azoreductase (AcpD) of Escherichia coli has been identified in this strain. The gene in E. faecalis, designated as azoA, encoded a protein of 208 amino acids with a calculated isoelectric point of 4.8. AzoA was heterologously overexpressed in E. coli with a strong band of 23 kDa on SDS-PAGE. The purified recombinant enzyme was a homodimer with a molecular weight of 43 kDa, probably containing one molecule of FMN per dimer. AzoA required FMN and NADH, but not NADPH, as a preferred electron donor for its activity. The apparent Km values for both NADH and 2-[4-(dimethylamino)phenylazo]benzoic acid (Methyl red) substrates were 0.14 and 0.024 mM, respectively. The apparent Vmax was 86.2 microM/min/mg protein. The enzyme was not only able to decolorize Methyl red, but was also able to convert sulfonated azo dyes Orange II, Amaranth, Ponceau BS, and Ponceau S. AzoA is the first aerobic azoreductase to be identified and characterized from human intestinal gram-positive bacteria.
Figures


References
-
- Chung K-T, Stevens SE, Jr, Cerniglia CE. The production of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18:175–190. - PubMed
-
- Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol. 2001;56:69–80. - PubMed
-
- Gingell R, Walker R. Mechanisms of azo reduction by Streptococcus faecalis. II. The role of soluble flavins. Xenobiotica. 1971;1:231–239. - PubMed
-
- Zimmermann T, Gasser F, Kulla HG, Leisinger T. Comparison of two bacterial azoreductases acquired during adaptation to growth on azo dyes. Arch Microbiol. 1984;138:37–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

