WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem
- PMID: 15004006
- PMCID: PMC359391
- DOI: 10.1101/gad.291204
WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem
Abstract
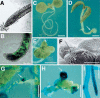
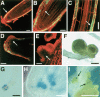
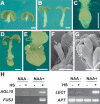
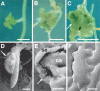
Most of the plant shoot originates f om a small group of stem cells, which in A abidopsis are specified by WUSCHEL (WUS). It is unknown whether these cells have an inrinsic potential to generate shoot tissues, or whether differentiation is guided by signals from more mature tissues. He e we show that WUS expression in the root induced shoot stem cell identity and leaf development (without additional cues), floral development (together with LEAFY), or embryogenesis (in response to increased auxin). Thus, WUS establishes stem cells with intrinsic shoot identity and responsive to developmental inputs that normally do not change root identity.
Figures

References
-
- Brand U., Fletcher, J.C., Hobe, M., Meyerowitz, E.M., and Simon, R. 2000. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619. - PubMed
-
- Clough S.J. and Bent, A.F. 1998. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. - PubMed
-
- Feher A., Pasternak, T.P., and Dudits, D. 2003. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 74: 201–228.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
