Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor
- PMID: 15004228
- PMCID: PMC404022
- DOI: 10.1091/mbc.e03-11-0839
Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor
Abstract
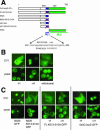
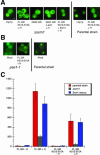
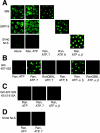
The vertebrate glucocorticoid receptor (GR) is cytoplasmic without hormone and localizes to the nucleus after hormone binding. GR has two nuclear localization signals (NLS): NL1 is similar in sequence to the SV40 NLS; NL2 is poorly defined, residing in the ligand-binding domain. We found that GR displayed similar hormone-regulated compartmentalization in Saccharomyces cerevisiae and required the Sxm1 nuclear import receptor for NL2-mediated import. Two metazoan homologues of Sxm1, importin 7 and importin 8, bound both NL1 and NL2, whereas importin alpha selectively bound NL1. In an in vitro nuclear import assay, both importin 7 and the importin alpha-importin beta heterodimer could import a GR NL1 fragment. Under these conditions, full-length GR localized to nuclei in the presence but not absence of an unidentified component in cell extracts. Interestingly, importin 7, importin 8, and importin alpha bound GR even in the absence of hormone; thus, hormonal control of localization is exerted at a step downstream of import receptor binding.
Figures




Similar articles
-
Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor.Mol Cell Biol. 1999 Feb;19(2):1025-37. doi: 10.1128/MCB.19.2.1025. Mol Cell Biol. 1999. PMID: 9891038 Free PMC article.
-
Nuclear Respiratory Factor 2β (NRF-2β) recruits NRF-2α to the nucleus by binding to importin-α:β via an unusual monopartite-type nuclear localization signal.J Mol Biol. 2013 Sep 23;425(18):3536-48. doi: 10.1016/j.jmb.2013.07.007. Epub 2013 Jul 13. J Mol Biol. 2013. PMID: 23856623
-
Evidence for importin alpha independent nuclear translocation of glucocorticoid receptors in Xenopus laevis oocytes.Cell Physiol Biochem. 2004;14(4-6):343-50. doi: 10.1159/000080344. Cell Physiol Biochem. 2004. PMID: 15319538
-
Classical nuclear localization signals: definition, function, and interaction with importin alpha.J Biol Chem. 2007 Feb 23;282(8):5101-5. doi: 10.1074/jbc.R600026200. Epub 2006 Dec 14. J Biol Chem. 2007. PMID: 17170104 Free PMC article. Review.
-
Molecular mechanisms of nuclear protein transport.Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review.
Cited by
-
Regulating Phase Transition in Neurodegenerative Diseases by Nuclear Import Receptors.Biology (Basel). 2022 Jul 4;11(7):1009. doi: 10.3390/biology11071009. Biology (Basel). 2022. PMID: 36101390 Free PMC article. Review.
-
The Nup153-Nup50 protein interface and its role in nuclear import.J Biol Chem. 2012 Nov 9;287(46):38515-22. doi: 10.1074/jbc.M112.378893. Epub 2012 Sep 24. J Biol Chem. 2012. PMID: 23007389 Free PMC article.
-
Identification and characterization of Drosophila Snurportin reveals a role for the import receptor Moleskin/importin-7 in snRNP biogenesis.Mol Biol Cell. 2013 Sep;24(18):2932-42. doi: 10.1091/mbc.E13-03-0118. Epub 2013 Jul 24. Mol Biol Cell. 2013. PMID: 23885126 Free PMC article.
-
A mechanism of nucleocytoplasmic trafficking for the homeodomain protein PRH.Mol Cell Biochem. 2009 Dec;332(1-2):173-81. doi: 10.1007/s11010-009-0188-0. Epub 2009 Jul 9. Mol Cell Biochem. 2009. PMID: 19588232 Free PMC article.
-
N-terminal domain of the androgen receptor contains a region that can promote cytoplasmic localization.J Steroid Biochem Mol Biol. 2014 Jan;139:16-24. doi: 10.1016/j.jsbmb.2013.09.013. Epub 2013 Oct 4. J Steroid Biochem Mol Biol. 2014. PMID: 24099702 Free PMC article.
References
-
- Aitchison, J.D., Blobel, G., and Rout, M.P. (1996). Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science 274, 624–627. - PubMed
-
- Black, B.E., Holaska, J.M., Rastinejad, F., and Paschal, B.M. (2001). DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr. Biol. 11, 1749–1758. - PubMed
-
- Dahlman-Wright, K., Siltala-Roos, H., Carlstedt-Duke, J., and Gustafsson, J.A. (1990). Protein-protein interactions facilitate DNA binding by the glucocorticoid receptor DNA-binding domain. J. Biol. Chem. 265, 14030–14035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

