The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins
- PMID: 15004235
- PMCID: PMC404034
- DOI: 10.1091/mbc.e03-09-0699
The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins
Abstract
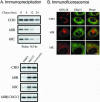
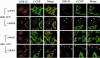
The conserved oligomeric Golgi (COG) complex is a soluble hetero-octamer associated with the cytoplasmic surface of the Golgi. Mammalian somatic cell mutants lacking the Cog1 (ldlB) or Cog2 (ldlC) subunits exhibit pleiotropic defects in Golgi-associated glycoprotein and glycolipid processing that suggest COG is involved in the localization, transport, and/or function of multiple Golgi processing proteins. We have identified a set of COG-sensitive, integral membrane Golgi proteins called GEARs (mannosidase II, GOS-28, GS15, GPP130, CASP, giantin, and golgin-84) whose abundances were reduced in the mutant cells and, in some cases, increased in COG-overexpressing cells. In the mutants, some GEARs were abnormally localized in the endoplasmic reticulum and were degraded by proteasomes. The distributions of the GEARs were altered by small interfering RNA depletion of epsilon-COP in wild-type cells under conditions in which COG-insensitive proteins were unaffected. Furthermore, synthetic phenotypes arose in mutants deficient in both epsilon-COP and either Cog1 or Cog2. COG and COPI may work in concert to ensure the proper retention or retrieval of a subset of proteins in the Golgi, and COG helps prevent the endoplasmic reticulum accumulation and degradation of some GEARs.
Figures





Similar articles
-
Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells.J Cell Biol. 2005 Feb 28;168(5):747-59. doi: 10.1083/jcb.200412003. Epub 2005 Feb 22. J Cell Biol. 2005. PMID: 15728195 Free PMC article.
-
Genetic analysis of the subunit organization and function of the conserved oligomeric golgi (COG) complex: studies of COG5- and COG7-deficient mammalian cells.J Biol Chem. 2005 Sep 23;280(38):32736-45. doi: 10.1074/jbc.M505558200. Epub 2005 Jul 28. J Biol Chem. 2005. PMID: 16051600
-
Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport.Traffic. 2010 Dec;11(12):1552-66. doi: 10.1111/j.1600-0854.2010.01123.x. Epub 2010 Oct 15. Traffic. 2010. PMID: 20874812
-
Multi-component protein complexes and Golgi membrane trafficking.J Biochem. 2005 Feb;137(2):109-14. doi: 10.1093/jb/mvi024. J Biochem. 2005. PMID: 15749823 Review.
-
ER-to-Golgi transport: COP I and COP II function (Review).Mol Membr Biol. 2003 Jul-Sep;20(3):197-207. doi: 10.1080/0968768031000122548. Mol Membr Biol. 2003. PMID: 12893528 Review.
Cited by
-
Structure of Golgi transport proteins.Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a007609. doi: 10.1101/cshperspect.a007609. Cold Spring Harb Perspect Biol. 2011. PMID: 21813399 Free PMC article. Review.
-
Sugary Logistics Gone Wrong: Membrane Trafficking and Congenital Disorders of Glycosylation.Int J Mol Sci. 2020 Jun 30;21(13):4654. doi: 10.3390/ijms21134654. Int J Mol Sci. 2020. PMID: 32629928 Free PMC article. Review.
-
Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells.J Cell Biol. 2005 Feb 28;168(5):747-59. doi: 10.1083/jcb.200412003. Epub 2005 Feb 22. J Cell Biol. 2005. PMID: 15728195 Free PMC article.
-
Maintaining order: COG complex controls Golgi trafficking, processing, and sorting.FEBS Lett. 2019 Sep;593(17):2466-2487. doi: 10.1002/1873-3468.13570. Epub 2019 Aug 16. FEBS Lett. 2019. PMID: 31381138 Free PMC article. Review.
-
Membrane detachment is not essential for COG complex function.Mol Biol Cell. 2018 Apr 15;29(8):964-974. doi: 10.1091/mbc.E17-11-0694. Epub 2018 Mar 30. Mol Biol Cell. 2018. PMID: 29467253 Free PMC article.
References
-
- Axelsson, M.A., Karlsson, N.G., Steel, D.M., Ouwendijk, J., Nilsson, T., and Hansson, G.C. (2001). Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 11, 633–644. - PubMed
-
- Barlowe, C. (2002). COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol. 417–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

