The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines
- PMID: 15004236
- PMCID: PMC404040
- DOI: 10.1091/mbc.e03-09-0634
The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines
Abstract
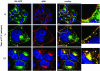
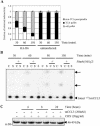
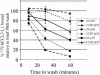
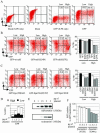
The D6 heptahelical membrane protein, expressed by lymphatic endothelial cells, is able to bind with high affinity to multiple proinflammatory CC chemokines. However, this binding does not allow D6 to couple to the signaling pathways activated by typical chemokine receptors such as CC-chemokine receptor-5 (CCR5). Here, we show that D6, like CCR5, can rapidly internalize chemokines. However, D6-internalized chemokines are more effectively retained intracellularly because they more readily dissociate from the receptor during vesicle acidification. These chemokines are then degraded while the receptor recycles to the cell surface. Interestingly, D6-mediated chemokine internalization occurs without bringing about a reduction in cell surface D6 levels. This is possible because unlike CCR5, D6 is predominantly localized in recycling endosomes capable of trafficking to and from the cell surface in the absence of ligand. When chemokine is present, it can enter the cells associated with D6 already destined for internalization. By this mechanism, D6 can target chemokines for degradation without the necessity for cell signaling, and without desensitizing the cell to subsequent chemokine exposure.
Figures






References
-
- Alcami, A. (2003). Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3, 36–50. - PubMed
-
- Bacon, K., et al. (2002). Chemokine/chemokine receptor nomenclature. J. Interferon Cytokine Res. 22, 1067–1068. - PubMed
-
- Barlic, J., et al. (1999). β-arrestins regulate interleukin-8-induced CXCR1 internalization. J. Biol. Chem. 274, 16287–16294. - PubMed
-
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

