Processing of clustered DNA damage generates additional double-strand breaks in mammalian cells post-irradiation
- PMID: 15004247
- PMCID: PMC390294
- DOI: 10.1093/nar/gkh306
Processing of clustered DNA damage generates additional double-strand breaks in mammalian cells post-irradiation
Abstract
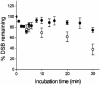
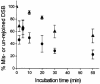
Clustered DNA damage sites, in which two or more lesions are formed within a few helical turns of the DNA after passage of a single radiation track, are signatures of DNA modifications induced by ionizing radiation in mammalian cells. Mutant hamster cells (xrs-5), deficient in non-homologous end joining (NHEJ), were irradiated at 37 degrees C to determine whether any additional double-strand breaks (DSBs) are formed during processing of gamma-radiation-induced DNA clustered damage sites. A class of non-DSB clustered DNA damage, corresponding to approximately 30% of the initial yield of DSBs, is converted into DSBs reflecting an artefact of preparation of genomic DNA for pulsed field gel electrophoresis. These clusters are removed within 4 min in both NHEJ-deficient and wild-type CHO cells. In xrs-5 cells, a proportion of non-DSB clustered DNA damage, representing approximately 10% of the total yield of non-DSB clustered DNA damage sites, are also converted into DSBs within approximately 30 min post-gamma but not post-alpha irradiation through cellular processing at 37 degrees C. That the majority of radiation-induced non-DSB clustered DNA damage sites are resistant to conversion into DSBs may be biologically significant at environmental levels of radiation exposure, as a non-DSB clustered damage site rather than a DSB, which only constitutes a minor proportion, is more likely to be induced in irradiated cells.
Figures
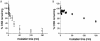

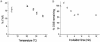
Similar articles
-
Increased repair of gamma-induced DNA double-strand breaks at lower dose-rate in CHO cells.Can J Physiol Pharmacol. 2004 Feb;82(2):125-32. doi: 10.1139/y04-006. Can J Physiol Pharmacol. 2004. PMID: 15052293
-
The role of nonhomologous DNA end joining, conservative homologous recombination, and single-strand annealing in the cell cycle-dependent repair of DNA double-strand breaks induced by H(2)O(2) in mammalian cells.Radiat Res. 2008 Dec;170(6):784-93. doi: 10.1667/RR1375.1. Radiat Res. 2008. PMID: 19138034
-
Extensive repair of DNA double-strand breaks in cells deficient in the DNA-PK-dependent pathway of NHEJ after exclusion of heat-labile sites.Radiat Res. 2009 Aug;172(2):152-64. doi: 10.1667/RR1745.1. Radiat Res. 2009. PMID: 19630520
-
Ionizing radiation and genetic risks XIV. Potential research directions in the post-genome era based on knowledge of repair of radiation-induced DNA double-strand breaks in mammalian somatic cells and the origin of deletions associated with human genomic disorders.Mutat Res. 2005 Oct 15;578(1-2):333-70. doi: 10.1016/j.mrfmmm.2005.06.020. Epub 2005 Aug 5. Mutat Res. 2005. PMID: 16084534 Review.
-
Radiation-induced clustered DNA lesions: Repair and mutagenesis.Free Radic Biol Med. 2017 Jun;107:125-135. doi: 10.1016/j.freeradbiomed.2016.12.008. Epub 2016 Dec 8. Free Radic Biol Med. 2017. PMID: 27939934 Review.
Cited by
-
DNA damage-associated biomarkers in studying individual sensitivity to low-dose radiation from cardiovascular imaging.Eur Heart J. 2016 Oct 21;37(40):3075-3080. doi: 10.1093/eurheartj/ehw206. Epub 2016 Jun 5. Eur Heart J. 2016. PMID: 27272147 Free PMC article. Review. No abstract available.
-
X-rays-Induced Bystander Effect Consists in the Formation of DNA Breaks in a Calcium-Dependent Manner: Influence of the Experimental Procedure and the Individual Factor.Biomolecules. 2023 Mar 16;13(3):542. doi: 10.3390/biom13030542. Biomolecules. 2023. PMID: 36979480 Free PMC article.
-
Probing Enhanced Double-Strand Break Formation at Abasic Sites within Clustered Lesions in Nucleosome Core Particles.Biochemistry. 2017 Jan 10;56(1):14-21. doi: 10.1021/acs.biochem.6b01144. Epub 2016 Dec 22. Biochemistry. 2017. PMID: 28005342 Free PMC article.
-
Delayed repair of radiation induced clustered DNA damage: friend or foe?Mutat Res. 2011 Jun 3;711(1-2):134-41. doi: 10.1016/j.mrfmmm.2010.11.003. Epub 2010 Dec 2. Mutat Res. 2011. PMID: 21130102 Free PMC article. Review.
-
The potential and hurdles of targeted alpha therapy - clinical trials and beyond.Front Oncol. 2014 Jan 14;3:324. doi: 10.3389/fonc.2013.00324. eCollection 2014 Jan 14. Front Oncol. 2014. PMID: 24459634 Free PMC article. Review.
References
-
- O'Neill P. and Fielden,E.M. (1993) Primary free radical processes in DNA. Adv. Radiat. Biol., 17, 53–120.
-
- Wallace S.S. (2002) Biological consequences of free radical-damaged DNA bases. Free Radic. Biol. Med., 33, 1–14. - PubMed
-
- von Sonntag C. (1987) The Chemical Basis of Radiation Biology. Taylor & Francis, Basingstoke, UK. ISBN 0-85066, p. 375.
-
- Johnston P.J., MacPhail,S.H., Stamato,T.D., Kirchgessner,C.U. and Olive,P. (1998) Higher-order chromatin structure-dependent repair of DNA double-strand breaks: involvement of the V(D)J recombination double-strand break repair pathway. Radiat. Res., 149, 455–462. - PubMed
-
- Jeggo P. (1990) Studies on mammalian mutants defective in rejoining double-strand breaks in DNA. Mutat. Res., 239, 1–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

