Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons
- PMID: 15004273
- PMCID: PMC374351
- DOI: 10.1073/pnas.0308118101
Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons
Abstract
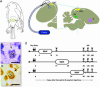
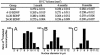
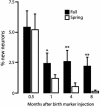
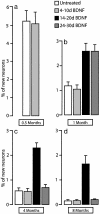
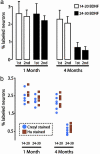
The high vocal center (HVC) of adult male canaries, Serinus canaria, is necessary for the production of learned song. New neurons are added to HVC every day, where they replace older neurons that have died, but the length of their survival depends on the time of year when they are born. A great number of HVC neurons born in the fall, when adult canaries learn a new song, are still present 8 mo later, when this song is used during the breeding season. By contrast, most of the neurons born in HVC in the spring, when little song learning takes place, disappear much sooner. Here we show that infusion of brain-derived neurotrophic factor into HVC during days 14-20 after new HVC neurons are born in the spring confers on them a life expectancy comparable to that of fall-born neurons; this extension on life is not seen when infusion occurs 10 days earlier or later. We suggest that there is, in the adult HVC, a subset of neurons whose life expectancy is determined by brain-derived neurotrophic factor during a sensitive period soon after these neurons reach destination and start forming connections.
Figures

Similar articles
-
A relationship between behavior, neurotrophin expression, and new neuron survival.Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8584-9. doi: 10.1073/pnas.140222497. Proc Natl Acad Sci U S A. 2000. PMID: 10890902 Free PMC article.
-
Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song.Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7844-8. doi: 10.1073/pnas.91.17.7844. Proc Natl Acad Sci U S A. 1994. PMID: 8058721 Free PMC article.
-
BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain.Neuron. 1999 Jan;22(1):53-62. doi: 10.1016/s0896-6273(00)80678-9. Neuron. 1999. PMID: 10027289
-
Birth, migration, incorporation, and death of vocal control neurons in adult songbirds.J Neurobiol. 1997 Nov;33(5):585-601. J Neurobiol. 1997. PMID: 9369461 Review.
-
A species-specific view of song representation in a sensorimotor nucleus.J Physiol Paris. 2013 Jun;107(3):193-202. doi: 10.1016/j.jphysparis.2012.08.004. Epub 2012 Aug 30. J Physiol Paris. 2013. PMID: 22960663 Review.
Cited by
-
Testosterone and brain-derived neurotrophic factor interactions in the avian song control system.Neuroscience. 2013 Jun 3;239:115-23. doi: 10.1016/j.neuroscience.2012.09.023. Epub 2012 Oct 30. Neuroscience. 2013. PMID: 23123886 Free PMC article. Review.
-
Adult neurogenesis is associated with the maintenance of a stereotyped, learned motor behavior.J Neurosci. 2012 May 16;32(20):7052-7. doi: 10.1523/JNEUROSCI.5385-11.2012. J Neurosci. 2012. PMID: 22593073 Free PMC article.
-
The zebra finch paradox: song is little changed, but number of neurons doubles.J Neurosci. 2012 Jan 18;32(3):761-74. doi: 10.1523/JNEUROSCI.3434-11.2012. J Neurosci. 2012. PMID: 22262875 Free PMC article.
-
Rapid seasonal-like regression of the adult avian song control system.Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15520-5. doi: 10.1073/pnas.0707239104. Epub 2007 Sep 17. Proc Natl Acad Sci U S A. 2007. PMID: 17875989 Free PMC article.
-
DARPP-32 distinguishes a subset of adult-born neurons in zebra finch HVC.J Comp Neurol. 2022 Apr;530(5):792-803. doi: 10.1002/cne.25245. Epub 2021 Nov 15. J Comp Neurol. 2022. PMID: 34545948 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

