Microtubule-kinesin interface mutants reveal a site critical for communication
- PMID: 15005614
- PMCID: PMC1543712
- DOI: 10.1021/bi035830e
Microtubule-kinesin interface mutants reveal a site critical for communication
Abstract
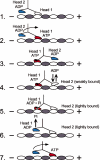
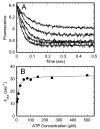
Strict coordination of the two motor domains of kinesin is required for driving the processive movement of organelles along microtubules. Glutamate 164 of the kinesin heavy chain was shown to be critical for kinesin function through in vivo genetics in Drosophila melanogaster. The mutant motor E164K exhibited reduced steady-state ATPase activity and higher affinity for both ATP and microtubules. Moreover, an alanine substitution at this position (E164A) caused similar defects. It became stalled on the microtubule and was unable to bind and hydrolyze ATP at the second motor domain. Glu(164), which has been conserved through evolution, is located at the motor-microtubule interface close to key residues on helix alpha12 of beta-tubulin. We explored further the contributions of Glu(164) to motor function using several site-directed mutant proteins: E164K, E164N, E164D, E164Q, and D165A. The results indicate that the microtubule-E164K complex can only bind and hydrolyze one ATP. ATP with increased salt was able to dissociate a population of E164K motors from the microtubule but could not dissociate E164A. We tested the basis of the stabilized microtubule interaction with E164K, E164N, and E164A. The results provide new insights about the motor-microtubule interface and the pathway of communication for processive motility.
Figures






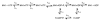
References
-
- Vale RD, Milligan RA. The way things move: Looking under the hood of molecular motor proteins. Science. 2000;288:88–95. - PubMed
-
- Schief WR, Howard J. Conformational changes during kinesin motility. Curr. Opin. Cell Biol. 2001;13:19–28. - PubMed
-
- Schliwa M, Woehlke G. Molecular motors. Nature. 2003;422:759–765. - PubMed
-
- Mandelkow E, Johnson KA. The structural and mechanochemical cycle of kinesin. Trends Bochem. Sci. 1998;23:429–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

