Detection of Arcobacter spp. in the coastal environment of the Mediterranean Sea
- PMID: 15006743
- PMCID: PMC368354
- DOI: 10.1128/AEM.70.3.1271-1276.2004
Detection of Arcobacter spp. in the coastal environment of the Mediterranean Sea
Abstract
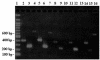
The occurrence of Arcobacter spp. was studied in seawater and plankton samples collected from the Straits of Messina, Italy, during an annual period of observation by using cultural and molecular techniques. A PCR assay with three pairs of primers targeting the 16S and 23S rRNA genes was used for detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii in cultures and environmental samples. Only one of the Arcobacter species, A. butzleri, was isolated from seawater and plankton samples. With some samples the A. butzleri PCR assay gave amplified products when cultures were negative. A. cryaerophilus and A. skirrowii were never detected by culture on selective agar plates; they were detected only by PCR performed directly with environmental samples. Collectively, our data suggest that culturable and nonculturable forms of Arcobacter are present in marine environments. The assay was useful for detecting Arcobacter spp. both as free forms and intimately associated with plankton. This is the first report showing both direct isolation of A. butzleri and the presence of nonculturable Arcobacter spp. in the coastal environment of the Mediterranean Sea.
Figures



References
-
- Barer, M. R., L. T. Gribbon, C. R. Harwood, and C. E. Nwoguh. 1993. The viable but non-culturable hypothesis and medical bacteriology. Rev. Med. Microbiol. 4:183-191.
-
- Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41:93-137. - PubMed
-
- Colwell, R. R., and H. Huq. 1994. Vibrios in the environment: viable but nonculturable Vibrio cholerae, p. 117-133. In I. K. Wachsmuth, P. A. Blake, and Ø. Olsvik (ed.), Vibrio cholerae and cholera: molecular global perspectives. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

