Phage community dynamics in hot springs
- PMID: 15006788
- PMCID: PMC368299
- DOI: 10.1128/AEM.70.3.1633-1640.2004
Phage community dynamics in hot springs
Abstract
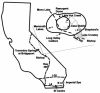
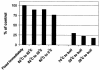
In extreme thermal environments such as hot springs, phages are the only known microbial predators. Here we present the first study of prokaryotic and phage community dynamics in these environments. Phages were abundant in hot springs, reaching concentrations of a million viruses per milliliter. Hot spring phage particles were resistant to shifts to lower temperatures, possibly facilitating DNA transfer out of these extreme environments. The phages were actively produced, with a population turnover time of 1 to 2 days. Phage-mediated microbial mortality was significant, making phage lysis an important component of hot spring microbial food webs. Together, these results show that phages exert an important influence on microbial community structure and energy flow in extreme thermal environments.
Figures



References
-
- Anderson, N. G. (ed.). 1967. Isolation of viral particles from large volumes. Interscience Publishers, New York, N.Y.
-
- Anderson, N. G., and G. B. Cline. 1967. New centrifugal methods for virus isolation, p. 137-178. In M. K. Maramorosch and H. Koprowski (ed.), Methods in virology, vol. II. Academic Press, New York, N.Y.
-
- Arnold, H. P., W. Zillig, U. Ziese, I. Holz, M. Crosby, T. Utterback, J. F. Weidmann, J. K. Kristjanson, H. P. Klenk, K. E. Nelson, and C. M. Fraser. 2000. A novel lipothrixvirus, SIFV, of the extremely thermophilic crenarchaeon Sulfolobus. Virology 267:252-266. - PubMed
-
- Azam, F. 1998. Microbial control of oceanic carbon flux: the plot thickens. Science 280:694-696.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

