Antisense RNA targeting of primase interferes with bacteriophage replication in Streptococcus thermophilus
- PMID: 15006799
- PMCID: PMC368297
- DOI: 10.1128/AEM.70.3.1735-1743.2004
Antisense RNA targeting of primase interferes with bacteriophage replication in Streptococcus thermophilus
Abstract
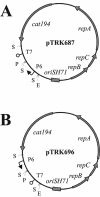
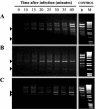
The putative primase gene and other genes associated with the Sfi21-prototype genome replication module are highly conserved in Streptococcus thermophilus bacteriophages. Expression of antisense RNAs complementary to the putative primase gene (pri3.1) from S. thermophilus phage kappa 3 provided significant protection from kappa 3 and two other Sfi21-type phages. Expression of pri3.10-AS, an antisense RNA that covered the entire primase gene, reduced the efficiency of plaquing (EOP) of kappa 3 to 3 x 10(-3) and reduced its burst size by 20%. Mutant phages capable of overcoming antisense inhibition were not recovered. Thirteen primase-specific antisense cassettes of different lengths (478 to 1,512 bp) were systematically designed to target various regions of the gene. Each cassette conferred some effect, reducing the EOP to between 0.8 and 3 x 10(-3). The largest antisense RNAs (1.5 kb) were generally found to confer the greatest reductions in EOP, but shorter (0.5 kb) antisense RNAs were also effective, especially when directed to the 5' region of the gene. The impacts of primase-targeted antisense RNAs on phage development were examined. The expression of pri3.10-AS resulted in reductions in target RNA abundance and the number of phage genomes synthesized. Targeting a key genome replication function with antisense RNA provided effective phage protection in S. thermophilus.
Figures

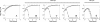

Similar articles
-
Expression of antisense RNA targeted against Streptococcus thermophilus bacteriophages.Appl Environ Microbiol. 2002 Feb;68(2):588-96. doi: 10.1128/AEM.68.2.588-596.2002. Appl Environ Microbiol. 2002. PMID: 11823195 Free PMC article.
-
The genetic relationship between virulent and temperate Streptococcus thermophilus bacteriophages: whole genome comparison of cos-site phages Sfi19 and Sfi21.Virology. 1999 Aug 1;260(2):232-43. doi: 10.1006/viro.1999.9814. Virology. 1999. PMID: 10417258
-
Inhibition of bacteriophage replication in Streptococcus thermophilus by subunit poisoning of primase.Microbiology (Reading). 2007 Oct;153(Pt 10):3295-3302. doi: 10.1099/mic.0.2007/007567-0. Microbiology (Reading). 2007. PMID: 17906129
-
Engineered bacteriophage-defence systems in bioprocessing.Nat Rev Microbiol. 2006 May;4(5):395-404. doi: 10.1038/nrmicro1393. Nat Rev Microbiol. 2006. PMID: 16715051 Review.
-
Molecular ecology and evolution of Streptococcus thermophilus bacteriophages--a review.Virus Genes. 1998;16(1):95-109. doi: 10.1023/a:1007957911848. Virus Genes. 1998. PMID: 9562894 Review.
Cited by
-
Determination of Technological Properties and CRISPR Profiles of Streptococcus thermophilus Isolates Obtained from Local Yogurt Samples.Microorganisms. 2024 Nov 25;12(12):2428. doi: 10.3390/microorganisms12122428. Microorganisms. 2024. PMID: 39770631 Free PMC article.
-
Characterization of the cro-ori region of the Streptococcus thermophilus virulent bacteriophage DT1.Appl Environ Microbiol. 2005 Mar;71(3):1237-46. doi: 10.1128/AEM.71.3.1237-1246.2005. Appl Environ Microbiol. 2005. PMID: 15746324 Free PMC article.
-
Evaluation of the metS and murB loci for antibiotic discovery using targeted antisense RNA expression analysis in Bacillus anthracis.Antimicrob Agents Chemother. 2007 May;51(5):1708-18. doi: 10.1128/AAC.01180-06. Epub 2007 Mar 5. Antimicrob Agents Chemother. 2007. PMID: 17339372 Free PMC article.
-
Genomic organization and molecular analysis of virulent bacteriophage 2972 infecting an exopolysaccharide-producing Streptococcus thermophilus strain.Appl Environ Microbiol. 2005 Jul;71(7):4057-68. doi: 10.1128/AEM.71.7.4057-4068.2005. Appl Environ Microbiol. 2005. PMID: 16000821 Free PMC article.
-
Use of an antisense RNA strategy to investigate the functional significance of Mn-catalase in the extreme thermophile Thermus thermophilus.J Bacteriol. 2004 Nov;186(22):7804-6. doi: 10.1128/JB.186.22.7804-7806.2004. J Bacteriol. 2004. PMID: 15516595 Free PMC article.
References
-
- Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226.
-
- Bouchard, J. D., and S. Moineau. 2000. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology 270:65-75. - PubMed
-
- Brüssow, H., and F. Desiere. 2001. Comparative phage genomics and the evolution of Siphoviridae: insights from dairy phages. Mol. Microbiol. 39:213-222. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

