Involvement of bradykinin, cytokines, sympathetic amines and prostaglandins in formalin-induced orofacial nociception in rats
- PMID: 15006904
- PMCID: PMC1574892
- DOI: 10.1038/sj.bjp.0705724
Involvement of bradykinin, cytokines, sympathetic amines and prostaglandins in formalin-induced orofacial nociception in rats
Abstract
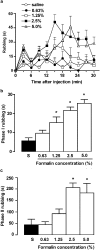
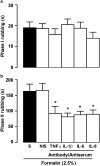
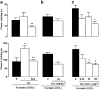
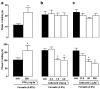
1. This study characterises some of the mechanisms and mediators involved in the orofacial nociception triggered by injection of formalin into the upper lip of the rat, by assessing the influence of various treatments on behavioural nociceptive responses (duration of facial rubbing) elicited either by a low subthreshold (i.e. non-nociceptive; 0.63%) or a higher concentration of the algogen (2.5%). 2. The kininase II inhibitor captopril (5 mg kg(-1), s.c.) and prostaglandin(PG) E(2) (100 ng lip(-1)) potentiated both phases of the response to 0.63% formalin, whereas tumour necrosis factor (TNF alpha; 5 pg lip(-1)), interleukin(IL)-1 beta (0.5 pg lip(-1)), IL-6 (2 ng lip(-1)) and IL-8 (200 pg lip(-1)), or the indirectly acting sympathomimetic drug tyramine (200 microg lip(-1)), each augmented only the second phase of nociception. 3. Conversely, both phases of nociception induced by 2.5% formalin were inhibited by the bradykinin (BK) B(2) receptor antagonist HOE140 (5 microg lip(-1)) or the selective beta(1)-adrenoceptor antagonist atenolol (100 microg lip(-1)). However, the BK B(1) receptor antagonist des-Arg(9)-Leu(8)-BK (1 and 2 microg lip(-1)), antibody and/or antiserum against each of the cytokines, the adrenergic neurone blocker guanethidine (30 mg kg(-1) day(-1), s.c., for 3 days) and the cyclooxygenase(COX)-2 inhibitor celecoxib (50 and 200 microg lip(-1), s.c.; or 1 and 3 mg kg(-1), i.p.) reduced only the second phase of the response. The nonselective COX inhibitor indomethacin and the 5-lipoxygenase activating protein inhibitor MK886 did not change formalin-induced nociception. 4. Our results indicate that BK, TNF-alpha, IL-1 beta, IL-6, IL-8, sympathetic amines and PGs (but not leukotrienes) contribute significantly to formalin-induced orofacial nociception in the rat and the response seems to be more susceptible to inhibition by B(2) receptor antagonist and selective COX-2 inhibitor than by B(1) receptor antagonist or nonselective COX inhibitor.
Figures


Similar articles
-
TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor.Eur J Pharmacol. 2007 Nov 14;573(1-3):221-9. doi: 10.1016/j.ejphar.2007.07.007. Epub 2007 Jul 13. Eur J Pharmacol. 2007. PMID: 17669394
-
Effect of a kinin B2 receptor antagonist on LPS- and cytokine-induced neutrophil migration in rats.Br J Pharmacol. 2003 May;139(2):271-8. doi: 10.1038/sj.bjp.0705236. Br J Pharmacol. 2003. PMID: 12770932 Free PMC article.
-
The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception.J Pain. 2006 Dec;7(12):908-14. doi: 10.1016/j.jpain.2006.04.010. J Pain. 2006. PMID: 17157777
-
The orofacial formalin test.Neurosci Biobehav Rev. 2004 Apr;28(2):219-26. doi: 10.1016/j.neubiorev.2003.12.003. Neurosci Biobehav Rev. 2004. PMID: 15172765 Review.
-
Bradykinin and inflammatory pain.Trends Neurosci. 1993 Mar;16(3):99-104. doi: 10.1016/0166-2236(93)90133-7. Trends Neurosci. 1993. PMID: 7681240 Review.
Cited by
-
Effect of pilocarpine on the formalin-induced orofacial pain in rats.Vet Res Forum. 2012 Spring;3(2):91-5. Vet Res Forum. 2012. PMID: 25653753 Free PMC article.
-
Medial prefrontal cortex diclofenac-induced antinociception is mediated through GPR55, cannabinoid CB1, and mu-opioid receptors of this area and periaqueductal gray.Naunyn Schmiedebergs Arch Pharmacol. 2020 Mar;393(3):371-379. doi: 10.1007/s00210-019-01735-x. Epub 2019 Oct 22. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31641818
-
Antinociceptive and anxiolytic-like effects of Lavandula angustifolia essential oil on rat models of orofacial pain.J Appl Oral Sci. 2023 Jan 6;30:e20220304. doi: 10.1590/1678-7757-2002-0304. eCollection 2023. J Appl Oral Sci. 2023. PMID: 36629536 Free PMC article.
-
The validation of Calophyllum brasiliense ("guanandi") uses in Brazilian traditional medicine as analgesic by in vivo antinociceptive evaluation and its chemical analysis.Naunyn Schmiedebergs Arch Pharmacol. 2017 Jul;390(7):733-739. doi: 10.1007/s00210-017-1366-3. Epub 2017 Apr 8. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28391533
-
The analgesic effect of rolipram is associated with the inhibition of the activation of the spinal astrocytic JNK/CCL2 pathway in bone cancer pain.Int J Mol Med. 2016 Nov;38(5):1433-1442. doi: 10.3892/ijmm.2016.2763. Epub 2016 Sep 30. Int J Mol Med. 2016. PMID: 28025994 Free PMC article.
References
-
- AMANN R., SCHULIGOI R., LANZ I., PESKAR B.A. Effect of a 5-lipoxygenase inhibitor on nerve growth factor-induced thermal hyperalgesia in the rat. Eur. J. Pharmacol. 1996;306:89–91. - PubMed
-
- BEICHE F., SCHEUERER S., BRUNE K., GEISSLINGER G., GOPPELT-STRUEBE M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. - PubMed
-
- BEUSENBERG F.D., HOOGSTENDEN H.C., BONTA I.L., VAN AMSTERDAN J.G. Cyclic AMP enhancing drugs modulate eicosanoid release from human alveolar macrophages. Life Sci. 1994;54:1269–1274. - PubMed
-
- BIANCHI M., PANERAI A.E. Effects of lornoxicam, piroxicam, and meloxicam in a model of thermal hindpaw hyperalgesia induced by formalin injection in rat tail. Pharmacol Res. 2002;45:101–105. - PubMed
-
- BODDEKE E.W.G.M. Involvement of chemokines in pain. Eur. J. Pharmacol. 2001;429:115–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

