Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes
- PMID: 15007060
- PMCID: PMC2172283
- DOI: 10.1083/jcb.200312018
Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes
Abstract
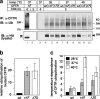
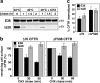
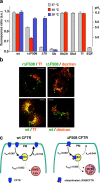
To investigate the degradation mechanism of misfolded membrane proteins from the cell surface, we used mutant cystic fibrosis transmembrane conductance regulators (CFTRs) exhibiting conformational defects in post-Golgi compartments. Here, we show that the folding state of CFTR determines the post-endocytic trafficking of the channel. Although native CFTR recycled from early endosomes back to the cell surface, misfolding prevented recycling and facilitated lysosomal targeting by promoting the ubiquitination of the channel. Rescuing the folding defect or down-regulating the E1 ubiquitin (Ub)-activating enzyme stabilized the mutant CFTR without interfering with its internalization. These observations with the preferential association of mutant CFTRs with Hrs, STAM-2, TSG101, hVps25, and hVps32, components of the Ub-dependent endosomal sorting machinery, establish a functional link between Ub modification and lysosomal degradation of misfolded CFTR from the cell surface. Our data provide evidence for a novel cellular mechanism of CF pathogenesis and suggest a paradigm for the quality control of plasma membrane proteins involving the coordinated function of ubiquitination and the Ub-dependent endosomal sorting machinery.
Figures
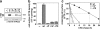




References
-
- Arvan, P., X. Zhao, J. Ramos-Castaneda, and A. Chang. 2002. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic. 3:771–780. - PubMed
-
- Benharouga, M., M. Sharma, J. So, M. Haardt, L. Drzymala, M. Popov, B. Schwapach, S. Grinstein, K. Du, and G.L. Lukacs. 2003. The role of the C terminus and Na+/H+ exchanger regulatory factor in the functional expression of cystic fibrosis transmembrane conductance regulator in nonpolarized cells and epithelia. J. Biol. Chem. 278:22079–22089. - PubMed
-
- Bilodeau, P., J. Urbanowski, S. Winistorfer, and R. Piper. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534–539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

