Keratins modulate colonocyte electrolyte transport via protein mistargeting
- PMID: 15007064
- PMCID: PMC2172274
- DOI: 10.1083/jcb.200308103
Keratins modulate colonocyte electrolyte transport via protein mistargeting
Abstract
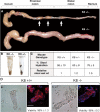
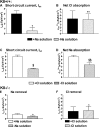
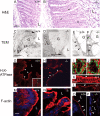
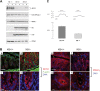
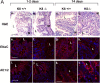
The function of intestinal keratins is unknown, although keratin 8 (K8)-null mice develop colitis, hyperplasia, diarrhea, and mistarget jejunal apical markers. We quantified the diarrhea in K8-null stool and examined its physiologic basis. Isolated crypt-units from K8-null and wild-type mice have similar viability. K8-null distal colon has normal tight junction permeability and paracellular transport but shows decreased short circuit current and net Na absorption associated with net Cl secretion, blunted intracellular Cl/HCO3-dependent pH regulation, hyperproliferation and enlarged goblet cells, partial loss of the membrane-proximal markers H,K-ATPase-beta and F-actin, increased and redistributed basolateral anion exchanger AE1/2 protein, and redistributed Na-transporter ENaC-gamma. Diarrhea and protein mistargeting are observed 1-2 d after birth while hyperproliferation/inflammation occurs later. The AE1/2 changes and altered intracellular pH regulation likely account, at least in part, for the ion transport defects and hyperproliferation. Therefore, colonic keratins have a novel function in regulating electrolyte transport, likely by targeting ion transporters to their cellular compartments.
Figures
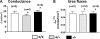
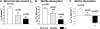
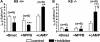

Similar articles
-
Keratin 8 knockdown leads to loss of the chloride transporter DRA in the colon.Am J Physiol Gastrointest Liver Physiol. 2016 Jun 1;310(11):G1147-54. doi: 10.1152/ajpgi.00354.2015. Epub 2016 Apr 28. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27125276 Free PMC article.
-
Basolateral ion transporters involved in colonic epithelial electrolyte absorption, anion secretion and cellular homeostasis.Acta Physiol (Oxf). 2011 Jan;201(1):33-46. doi: 10.1111/j.1748-1716.2010.02153.x. Acta Physiol (Oxf). 2011. PMID: 20528802 Review.
-
The amount of keratins matters for stress protection of the colonic epithelium.PLoS One. 2015 May 22;10(5):e0127436. doi: 10.1371/journal.pone.0127436. eCollection 2015. PLoS One. 2015. PMID: 26000979 Free PMC article.
-
Keratin 8 absence down-regulates colonocyte HMGCS2 and modulates colonic ketogenesis and energy metabolism.Mol Biol Cell. 2015 Jun 15;26(12):2298-310. doi: 10.1091/mbc.E14-02-0736. Epub 2015 Apr 22. Mol Biol Cell. 2015. PMID: 25904331 Free PMC article.
-
Bicarbonate secretion: a neglected aspect of colonic ion transport.J Clin Gastroenterol. 2005 Apr;39(4 Suppl 2):S53-8. doi: 10.1097/01.mcg.0000155521.81382.3a. J Clin Gastroenterol. 2005. PMID: 15758660 Review.
Cited by
-
Pathophysiology of IBD associated diarrhea.Tissue Barriers. 2018;6(2):e1463897. doi: 10.1080/21688370.2018.1463897. Epub 2018 May 8. Tissue Barriers. 2018. PMID: 29737913 Free PMC article. Review.
-
Placental vasculogenesis is regulated by keratin-mediated hyperoxia in murine decidual tissues.Am J Pathol. 2011 Apr;178(4):1578-90. doi: 10.1016/j.ajpath.2010.12.055. Am J Pathol. 2011. PMID: 21435445 Free PMC article.
-
Keratin 8 knockdown leads to loss of the chloride transporter DRA in the colon.Am J Physiol Gastrointest Liver Physiol. 2016 Jun 1;310(11):G1147-54. doi: 10.1152/ajpgi.00354.2015. Epub 2016 Apr 28. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27125276 Free PMC article.
-
Keratins and protein synthesis: the plot thickens.J Cell Biol. 2009 Oct 19;187(2):157-9. doi: 10.1083/jcb.200909134. J Cell Biol. 2009. PMID: 19841134 Free PMC article.
-
Intestinal intermediate filament polypeptides in C. elegans: Common and isotype-specific contributions to intestinal ultrastructure and function.Sci Rep. 2020 Feb 21;10(1):3142. doi: 10.1038/s41598-020-59791-w. Sci Rep. 2020. PMID: 32081918 Free PMC article.
References
-
- Ahn, Y.J., D.R. Brooker, F. Kosari, B.J. Harte, J. Li, S.A. Mackler, and T.R. Kleyman. 1999. Cloning and functional expression of the mouse epithelial sodium channel. Am. J. Physiol. 277:F121–F129. - PubMed
-
- Alper, S.L., R.B. Darman, M.N. Chernova, and N.K. Dahl. 2002. The AE gene family of Cl/HCO3- exchangers. J. Nephrol. 15(Suppl 5):S41–S53. - PubMed
-
- Alvarez de la Rosa, D., C.M. Canessa, G.K. Fyfe, and P. Zhang. 2000. Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 62:573-594. - PubMed
-
- Ameen, N.A., Y. Figueroa, and P.J. Salas. 2001. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell Sci. 114:563–575. - PubMed
-
- Baribault, H., J. Penner, R.V. Iozzo, and M. Wilson-Heiner. 1994. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 8:2964–2973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

