Lysophosphatidic acid induces neointima formation through PPARgamma activation
- PMID: 15007093
- PMCID: PMC2212723
- DOI: 10.1084/jem.20031619
Lysophosphatidic acid induces neointima formation through PPARgamma activation
Abstract
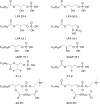
Neointimal lesions are characterized by accumulation of cells within the arterial wall and are a prelude to atherosclerotic disease. Here we report that a brief exposure to either alkyl ether analogs of the growth factor-like phospholipid lysophosphatidic acid (LPA), products generated during the oxidative modification of low density lipoprotein, or to unsaturated acyl forms of LPA induce progressive formation of neointima in vivo in a rat carotid artery model. This effect is completely inhibited by the peroxisome proliferator-activated receptor (PPAR)gamma antagonist GW9662 and mimicked by PPARgamma agonists Rosiglitazone and 1-O-hexadecyl-2-azeleoyl-phosphatidylcholine. In contrast, stearoyl-oxovaleryl phosphatidylcholine, a PPARalpha agonist and polypeptide epidermal growth factor, platelet-derived growth factor, and vascular endothelial growth factor failed to elicit neointima. The structure-activity relationship for neointima induction by LPA analogs in vivo is identical to that of PPARgamma activation in vitro and disparate from that of LPA G protein-coupled receptor activation. Neointima-inducing LPA analogs up-regulated the CD36 scavenger receptor in vitro and in vivo and elicited dedifferentiation of cultured vascular smooth muscle cells that was prevented by GW9662. These results suggest that selected LPA analogs are important novel endogenous PPARgamma ligands capable of mediating vascular remodeling and that activation of the nuclear transcription factor PPARgamma is both necessary and sufficient for neointima formation by components of oxidized low density lipoprotein.
Figures







References
-
- Steinberg, D. 2002. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat. Med. 8:1211–1217. - PubMed
-
- Dzau, V.J., R.C. Braun-Dullaeus, and D.G. Sedding. 2002. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 8:1249–1256. - PubMed
-
- Ross, R. 1999. Atherosclerosis is an inflammatory disease. Am. Heart J. 138:S419–S420. - PubMed
-
- Libby, P. 2002. Inflammation in atherosclerosis. Nature. 420:868–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

