Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN
- PMID: 15007094
- PMCID: PMC2212721
- DOI: 10.1084/jem.20031591
Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN
Abstract
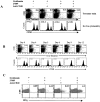
Toll-like receptors are important in the activation of innate immunity, and CD40 is a molecule critical for many T and B cell responses. Whereas agonists for either pathway have been used as vaccine adjuvants, we show that a combination of Toll-like receptor (TLR)7 and CD40 agonists synergize to stimulate CD8+ T cell responses 10-20-fold greater than the use of either agonist alone. Antigen-specific CD8+ T cells elicited from combination CD40/TLR7 treatment demonstrated both lytic activities and interferon (IFN)gamma production and an enhanced secondary response to antigenic challenge. Agonists for TLRs 2/6, 3, 4, and 9 also synergized with CD40 stimulation, demonstrating that synergy with the CD40 pathway is a property of TLR-derived stimuli in general. The CD8+ T cell expansion induced by CD40/TLR7 triggering was independent of CD4+ T cells, IFNgamma, and IL-12 but dependent on B7-mediated costimulation and surprisingly on type I IFN. These studies provide the rational basis for the use of TLR and CD40 agonists together as essential adjuvants to optimize vaccines designed to elicit protective or therapeutic immunity.
Figures


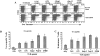


References
-
- Medzhitov, R., P. Preston-Hurlburt, and C.A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. - PubMed
-
- Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5–11. - PubMed
-
- Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. - PubMed
-
- Yamamoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, et al. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 420:324–329. - PubMed
-
- Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R.T. Schoen, R. Medzhitov, E. Fikrig, and R.A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878–884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

